100% Original 200ul Pipette tip - Eight-channel Mechanical Pipette – Bioendo
100% Original 200ul Pipette tip - Eight-channel Mechanical Pipette – Bioendo Detail:
Eight-Channel Mechanical Pipettor
1. Product information
All multi-channel mechanical pipettor have been quality tested according to ISO8655-2:2002 with calibration certificate. The quality control involves gravimetric testing of each pipette with distilled water at 22℃. The multichannel mechanical pipettor is idea for the detectionof bacterial endotoxin lal endotoxin testing by kinetic turbidimetric andkinetic chromogenic method.
- Eight-Channel Mechanical Pipettor is available for standard 96-well plate
- Dispensing head rotates for optimum pipetting convenience
- Individual piston and tip cone assemblies allow easy repair and maintenance
- Compound material tip cone design allows visual seal verification
- Can be used with universal style pipette tips
-good for kinetic chromogenic, kinetic turbidimetric TAL orend-point chromogenic TAL endotoxin assay
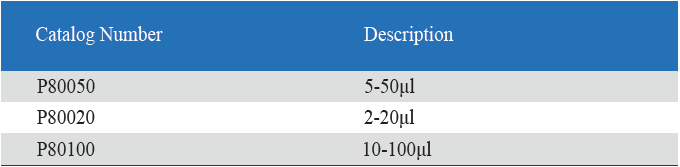
Product detail pictures:

Related Product Guide:
The corporate upholds the philosophy of "Be No.1 in excellent, be rooted on credit rating and trustworthiness for growth", will keep on to serve outdated and new clients from home and abroad whole-heatedly for 100% Original 200ul Pipette tip - Eight-channel Mechanical Pipette – Bioendo , The product will supply to all over the world, such as: Puerto Rico, Rome, Philadelphia, We've been adhering to the philosophy of "attracting customers with the best items and excellent service". We welcome customers, business associations and friends from all parts of the world to contact us and seek cooperation for mutual benefits.
Although we are a small company, we are also respected. Reliable quality, sincere service and good credit, we are honored to be able to work with you!