2022 China New Design Gel Clot Method - Bioendo GC Endotoxin Test Kit (Gel Clot Assay) – Bioendo
2022 China New Design Gel Clot Method - Bioendo GC Endotoxin Test Kit (Gel Clot Assay) – Bioendo Detail:
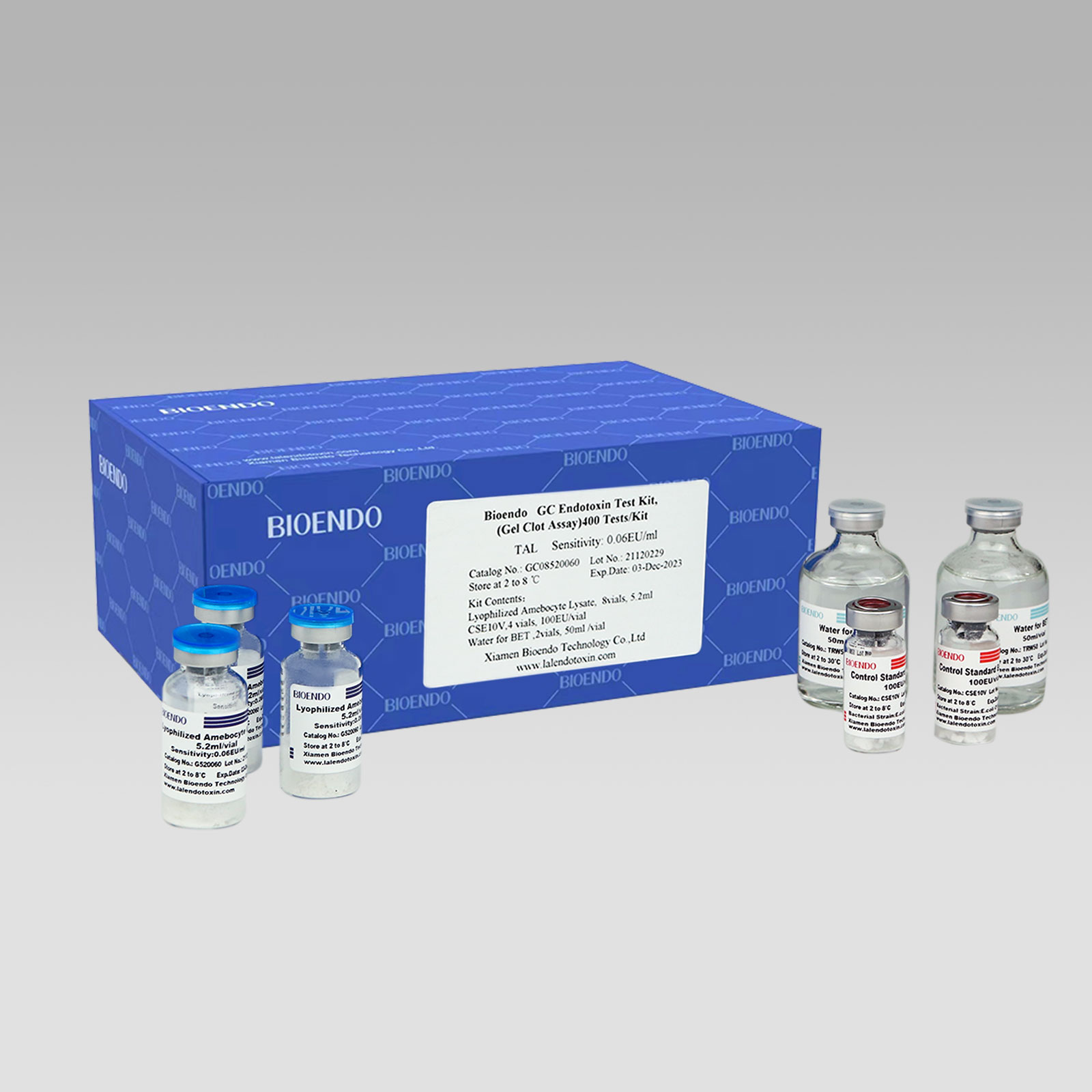
Bioendo GC Endotoxin Test Kit
Bioendo GC Endotoxin Test Kit (Gel Clot Assay)
1. Product Information
Bioendo GC Endotoxin Test Kit (Gel Clot Assay) contains 128 Tests/Kit, 400Tests/Kit, 1600Tests/Kit, and 4500 Tests/Kit. And sensitivity of Amebocyte Lysate included by the Kit is 0.03 EU/ml, 0.06 EU/ml, 0.125 EU/ml, 0.25 EU/ml, 0.5 EU/ml. The Kit contains Amebocyte Lysate, CSE and Water for BET. Four kinds of configuration of gel-clot endotoxin assay kit to satisfied the demand. This means you could choose different configuration kits according to the requirement.
2. Product Parameter
Sensitivities: 0.03EU/ml, 0.06EU/ml, 0.125EU/ml, 0.25EU/ml, 0.5 EU/ml
3.Product Feature and Application
Full range of sensitivities to meet the demand. No special instrument is needed for endotoxin detection with Bioendo GC Endotoxin Test Kit (Gel Clot Assay). It is the best choice when you need to do lots of endotoxin detection.
Note:
Lyophilized Amebocyte Lysate (LAL/TAL) reagent manufactured by Bioendo is made from amebocyte lysate derived blood of horseshoe crab.
|
Catalog No. |
Sensitivity (EU/ml) |
Description |
Kit Contents |
|
GC90520030 |
0.03 |
Bioendo™ GC Endotoxin Test Kit (Gel Clot Assay) 4500 Tests/Kit |
90 Gel Clot Lyophilized Amebocyte Lysate, 5.2ml (50 Tests/Vial); 20 CSE10V; |
|
GC90520060 |
0.06 |
||
|
GC90520125 |
0.125 |
||
|
GC90520250 |
0.25 |
||
|
GC90520500 |
0.5 |
||
|
GC08520030 |
0.03 |
Bioendo™ GC Endotoxin Test Kit (Gel Clot Assay) 400 Tests/Kit |
8 Gel Clot Lyophilized Amebocyte Lysate, 5.2ml (50 Tests/Vial); 4 CSE10V; 2 Water for BET, 50ml/vial; |
|
GC08520060 |
0.06 |
||
|
GC08520125 |
0.125 |
||
|
GC08520250 |
0.25 |
||
|
GC08520500 |
0.5 |
||
|
GC100170030 |
0.03 |
Bioendo™ GC Endotoxin Test Kit (Gel Clot Assay) 1600 Tests/Kit |
100 Gel Clot Lyophilized Amebocyte Lysate, 1.7ml (16 Tests/Vial); 10 CSE10V; |
|
GC100170060 |
0.06 |
||
|
GC100170125 |
0.125 |
||
|
GC100170250 |
0.25 |
||
|
GC100170500 |
0.5 |
||
|
GC08170030 |
0.03 |
Bioendo™ GC Endotoxin Test Kit (Gel Clot Assay) 128 Tests/Kit |
8 Gel Clot Lyophilized Amebocyte Lysate, 1.7ml (16 Tests/Vial); 4 CSE10V; 2 Water for BET, 50ml/vial; |
|
GC08170060 |
0.06 |
||
|
GC08170125 |
0.125 |
||
|
GC08170250 |
0.25 |
||
|
GC08170500 |
0.5 |
Product condition:
Endotoxin Test kit’s sensitivity and the Control Standard Endotoxin potency are assayed against USP Reference Standard Endotoxin. The Endotoxin Test reagentkits come with product instruction, Certificate of Analysis.
Product detail pictures:
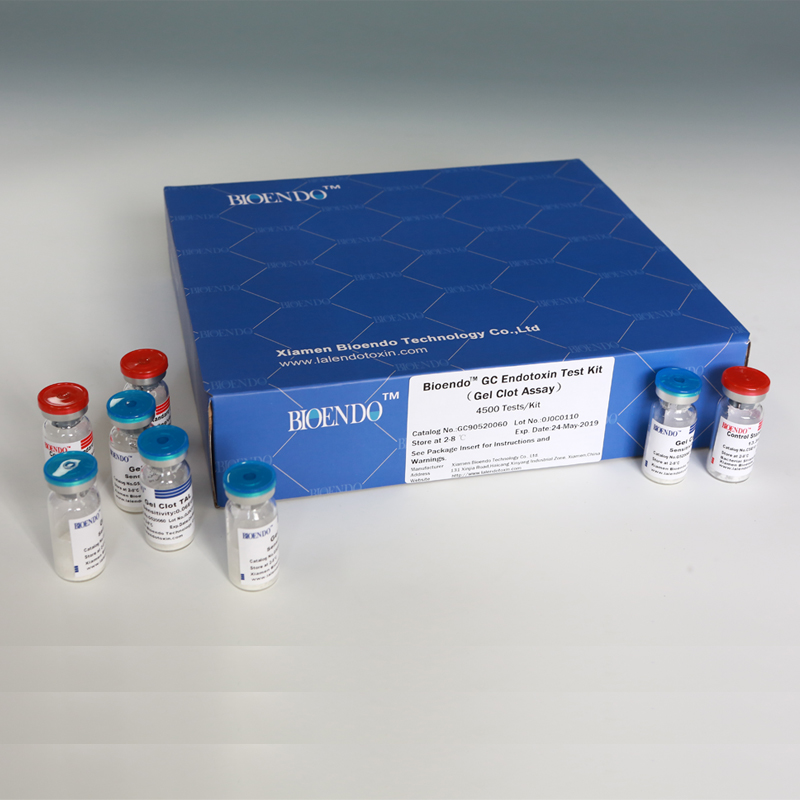

Related Product Guide:
"Quality initial, Honesty as base, Sincere support and mutual profit" is our idea, so as to build repeatedly and pursue the excellence for 2022 China New Design Gel Clot Method - Bioendo GC Endotoxin Test Kit (Gel Clot Assay) – Bioendo , The product will supply to all over the world, such as: Bahamas, Swiss, Peru, We warmly welcome your patronage and will serve our clients both at home and abroad with products and solutions of superior quality and excellent service geared to the trend of further development as always. We believe you will benefit from our professionalism soon.
This is the first business after our company establish, products and services are very satisfying, we have a good start, we hope to cooperate continuous in the future!