2022 China New Design Gel Clot Method - Gel Clot Lyophilized Amebocyte Lysate Multi-test Vial G52 – Bioendo
2022 China New Design Gel Clot Method - Gel Clot Lyophilized Amebocyte Lysate Multi-test Vial G52 – Bioendo Detail:
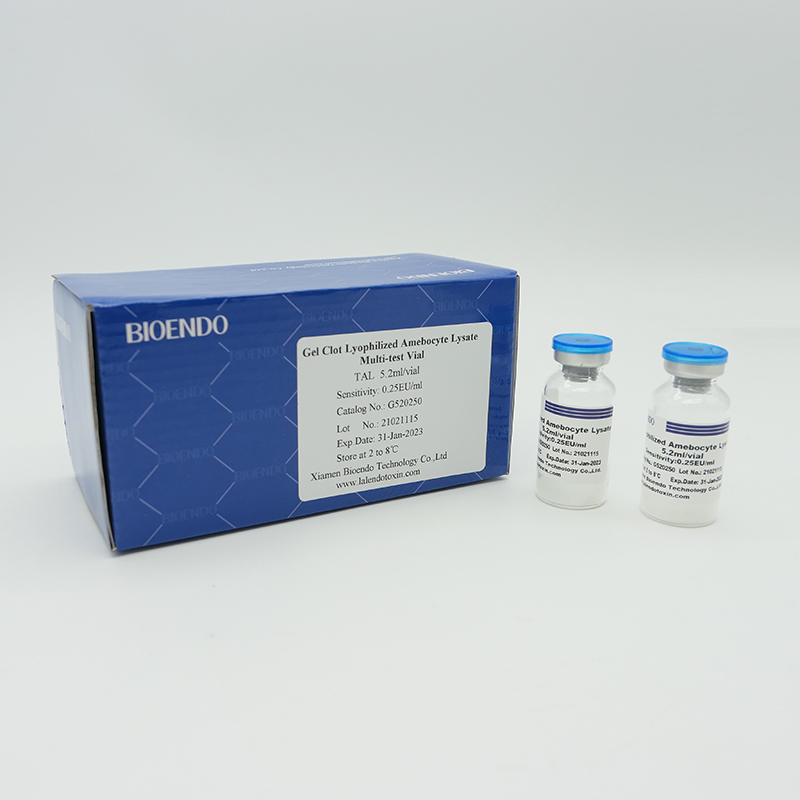
Gel Clot Method Lyophilized Amebocyte Lysate Multi-test Vial G52 series
1. Product Information
Gel Clot method Lyophilized Amebocyte Lysate Multi-test Vial is the Lyophilized Amebocyte Lysate reagent which select and use gel clot technique to detect endotoxin or pyrogen.
As the widespread method, gel-clot test for endotoxin is simple and does not require specific and expensive instrument. Bioendo provides Gel Clot Lyophilized Amebocyte Lysate reagent in 5.2ml per vial.
2. Product Parameters
Sensitivity range: 0.03EU/ml, 0.06EU/ml, 0.125EU/ml, 0.25EU/ml, 0.5 EU/ml
3. Product Application
End-product endotoxin (pyrogen) qualification, waterfor injection endotoxin assay, raw material endotoxin testing or endotoxinlevel monitoring during manufacturing process for pharmaceutical companies ormedical devices manufacturers.
Note:
Lyophilized Amebocyte Lysate (LAL) reagent manufactured by Bioendo is made from lysate of amebocytes (white blood cells) from the horseshoe crab.
| Catalog Number | Sensitivity (EU/ml or IU/ml) | ml/vial | Tests/Vial | Vials/Pack |
| G520030 | 0.03 | 5.2 | 50 | 10 |
| G520060 | 0.06 | 5.2 | 50 | 10 |
| G520125 | 0.125 | 5.2 | 50 | 10 |
| G520250 | 0.25 | 5.2 | 50 | 10 |
| G520500 | 0.5 | 5.2 | 50 | 10 |
Product condition:
The Lyophilized Amebocyte Lysate reagent sensitivity and the Control Standard Endotoxin potency are assayed against USP Reference Standard Endotoxin. The Lyophilized Amebocyte reagent kits come with product instruction, Certificate of Analysis, MSDS.
What is the difference between single test and multiple test?
● Single test: reconstitute the single test lysate reagent by BET water in the glass vial or glass ampoule.
● Multi-test: reconstitute the lysate reagent with BET water, and then add marked amount of lysate reagent following COA to the reaction tube or well plate for use. There is no difference in the sample pre-processing procedure; according to the amount of testing used, the sample size used for a single test is larger than the sample size used for multiple tests.
Why the most selected gel clot assay kit G52:
1. Multi test reagent for endotoxin detection in the applications of certain samples’ endotoxin detection.
2. G52 series of Gel clot assay multi test glass vial no need sophisticated microplate reader. Procedure of incubation by water bath or dry heat incubator.
3. High end quality of endotoxin free tube (<0.005EU/ml) and High quality of pyrogen free tips (<0.005EU/ml) as the guaranteed consumables to ensure the correct result.
Product detail pictures:



Related Product Guide:
Sticking to the principle of "Super Quality, Satisfactory service" ,We are striving to be a good business partner of you for 2022 China New Design Gel Clot Method - Gel Clot Lyophilized Amebocyte Lysate Multi-test Vial G52 – Bioendo , The product will supply to all over the world, such as: Kuwait, Netherlands, Rwanda, As operation principle is "be market-oriented , good faith as principle, win-win as objective", holding on "customer first, quality assurance, service first" as our purpose, dedicated to provide the original quality, create excellence service , we won the praise and trust in the industry of auto parts. In the future, We will provide quality product and excellent service in return to our customers , welcome any suggestions and feedback from all over the world.
On this website, product categories is clear and rich, I can find the product I want very quickly and easily, this is really very good!







