2022 High quality LAL assay kit - Mini Dry Heat Incubator – Bioendo
2022 High quality LAL assay kit - Mini Dry Heat Incubator – Bioendo Detail:
Dry heat incubator single module
1. Product information
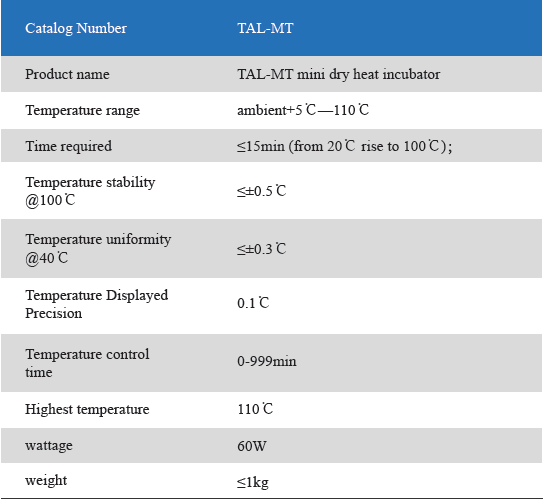
The Mini Dry Heat Incubator is a micro-processor controlled heating block with semi conductor heating technology.It adapts onboard use, smart, light and convenient for movement, suit for any kind of occasions. Especially good for the incubation of the gel clot LAL assay, LAL chromogenic endpoint assay incubation.
2. Product features
1. Unique designed. Smart and light, convenient movement, suit for various occasions.
2. LCD simultaneously display setting and actual time and temperature.Temperature calibration function.
3. Automatic fault detection function with buzzer alarm.
4. 24V DC input power, built-in over-temperature protection device.
5. Various of blocks for optional choice. Convenient for replacement. Easy cleaning and disinfection.
Product detail pictures:



Related Product Guide:
We take pleasure in an exceptionally excellent status between our buyers for our superb merchandise good quality, aggressive price tag and the greatest support for 2022 High quality LAL assay kit - Mini Dry Heat Incubator – Bioendo , The product will supply to all over the world, such as: Belgium, Kazakhstan, Bhutan, We follow superior mechanism to process these goods that ensure optimum durability and reliability of the goods. We follow latest effective washing and straightening processes that enable us to supply unmatched quality of items for our clients. We continually strive for perfection and all our efforts are directed towards attaining complete client satisfaction.
Although we are a small company, we are also respected. Reliable quality, sincere service and good credit, we are honored to be able to work with you!







