2022 High quality LAL assay kit - Single-Channel Mechanical Pipettor – Bioendo
2022 High quality LAL assay kit - Single-Channel Mechanical Pipettor – Bioendo Detail:
Single-Channel Mechanical Pipettor
1. Product Information
Single channel mechanical pipette is ideal tool to support endotoxin detection with Lyophilized Amebocyte Lysate which covers gel-clot technique, kinetic turbidimetric technique, kinetic chromogenic technique, and end-point chromogenic technique. All pipettors are produced by following ISO8655 – 2:2002. The quality control involves gravimetric testing of each pipette with distilled water at 22℃.
2. Product Features:
- Light weight, economic, low force design
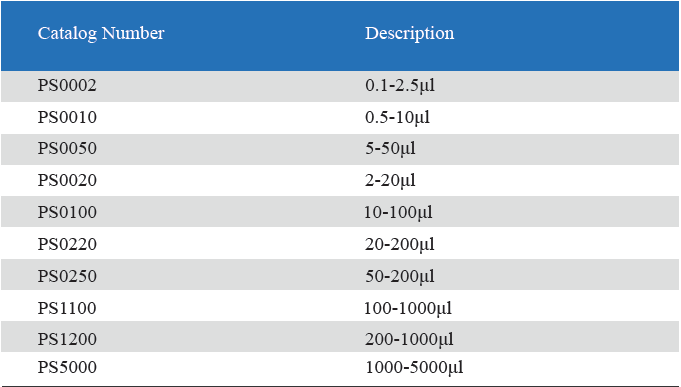
- Measuring volume range from 0.1μL to 5mL
- Easy to calibrate and maintain with tool supplied
- Design helps to avoid repetitive strain injuries
- Calibrated in accordance with ISO8655. Each pipettor supplied with individual test certificate
- The low part is available for autoclaving
Product detail pictures:

Related Product Guide:
Always customer-oriented, and it's our ultimate goal to get not only by far the most reputable, trustable and honest supplier, but also the partner for our customers for 2022 High quality LAL assay kit - Single-Channel Mechanical Pipettor – Bioendo , The product will supply to all over the world, such as: Cancun, Algeria, Madrid, Our company promises: reasonable prices, short production time and satisfactory after-sales service, we also welcome you to visit our factory at any time you want. Wish we have a pleasant and long terms business together!!!
High Quality, High Efficiency, Creative and Integrity, worth having long-term cooperation! Looking forward to the future cooperation!