2022 High quality LAL gel clot test - Gel Clot Lyophilized Amebocyte Lysate Single Test in Ampoule – Bioendo
2022 High quality LAL gel clot test - Gel Clot Lyophilized Amebocyte Lysate Single Test in Ampoule – Bioendo Detail:
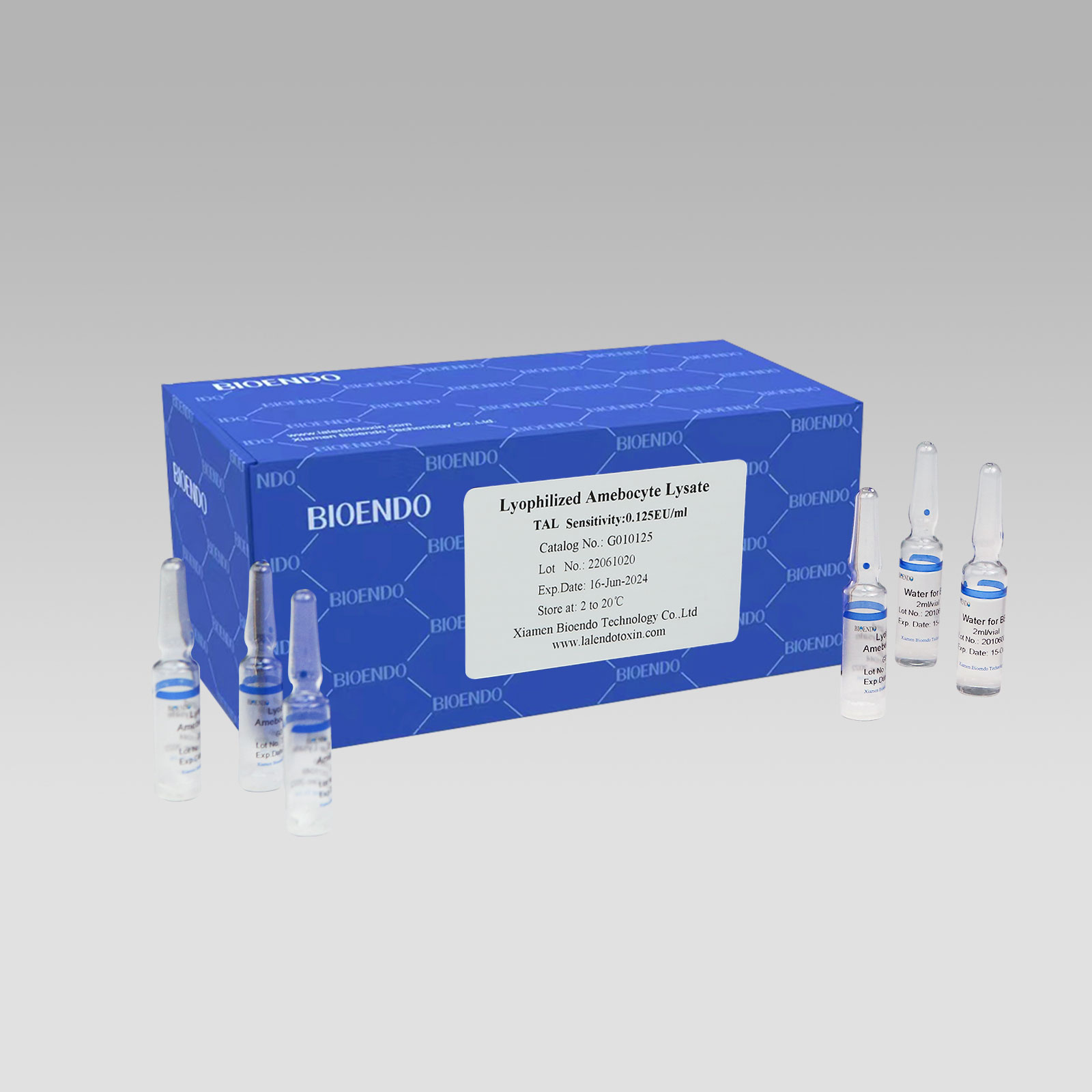
Gel Clot Lyophilized Amebocyte Lysate Single Test Kit (Glass Ampoule G01)
1. Product Information
Gel Clot Lyophilized Amebocyte Lysate Single Test in ampoule Contain endotoxin-specific Amebocyte Lysate which includes beta-glucan inhibitor in the formulation and will not react to beta-glucan. For our Single Test in glass ampoule, you could add sample to the glass ampoules directly. This means you don’t need to reconstitute Amebocyte Lysate in advance, and you will decide how many tests use each time to avoid waste, also this is the main difference from the endotoxin testing multi test vial. Endotoxin free tubes for the dilution of Lyophilized CSE is necessary. Operation of endotoxin detection use Bioendo Gel Clot Lyophilized Amebocyte Lysate Single Test in ampoule conforms to the national Pharmacopoeia.
2. Product Parameter
Gel clot assay single test glass ampoule.
Sensitivities: 0.03EU/ml, 0.06EU/ml, 0.125EU/ml, 0.25 EU/ml, 0.5EU/ml.
3.Product Feature and Application
Single step endotoxin detection, easy-to-use in the experiment of bacterial endotoxin test.
Normal water bath or dry heat incubator is available for the incubation of gel clot method LAL reagent.
Suitable for end-product endotoxin testing before product released.
Operate the endotoxin test assay, the kits sensitivity standardized according to the China Pharmacopoeia criterion.
Note:
Lyophilized Amebocyte Lysate (LAL) reagent manufactured by Bioendo is made from lysate of amebocytes (white blood cells) from the horseshoe crab.
|
Catalog No. |
Sensitivity EU/ml |
Description |
Kit Contents |
|
G010030 |
0.03 |
Bioendo Gel Clot Endotoxin Test Kit, Single Test in Ampoule. |
50 tests per pack |
|
G010060 |
0.06 |
||
|
G010125 |
0.125 |
||
|
G010250 |
0.25 |
||
|
G010500 |
0.5 |
Product Condition
The sensitivity of Lyophilized Amebocyte Lysate and potency of Control Standard Endotoxin are assayed against USP Reference Standard Endotoxin. The Lyophilized Amebocyte Lysate reagent kits come with product instruction, Certificate of Analysis.
Why the most selected gel clot assay kit G01:
1. The most economical and commonly used test reagent for endotoxin detection.
2. Single test in a ampoule for one step reduce the risk of contamination.
3. Gel clot assay single test glass ampoule no need sophisticated microplate reader.
4. Saving endotoxin free tube when using G01 series to test endotoxins in the procedure of endotoxin test assay’s operation.
Related products in the endotoxin test assay:
Water for Bacterial Endotoxins Test (BET), Recommend TRW02, TRW50 or TRW100
Endotoxin free glass tube ( dilution tube ), Recommend T1310018
Pyrogen free tips, Recommend PT25096 or PT100096
Pipettor, Recommend PSB0220
Test Tube Rack,
Incubation Instrument (Water Bath or Dry Heat Incubator ), Recommend Dry Heat Incubator TAL-M2
Vortex Mixter, Recommend VXH.
Control Standard Endotoxin, CSE10A.
Product detail pictures:

Related Product Guide:
Our products are greatly acknowledged and reliable by users and may fulfill repeatedly shifting financial and social wants for 2022 High quality LAL gel clot test - Gel Clot Lyophilized Amebocyte Lysate Single Test in Ampoule – Bioendo , The product will supply to all over the world, such as: Estonia, Uganda, Luxembourg, Our company always concentrate on the development of the international market. We've got a lot of customers in Russia , European countries, the USA, the Middle East countries and Africa countries. We always follow that quality is foundation while service is guarantee to meet all customers.
This supplier's raw material quality is stable and reliable, has always been in accordance with the requirements of our company to provide the goods that quality meet our requirements.