2022 wholesale price TAL assay - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo
2022 wholesale price TAL assay - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo Detail:
Single-Channel Mechanical Pipettes (Semi-sterile)
1. Product Information
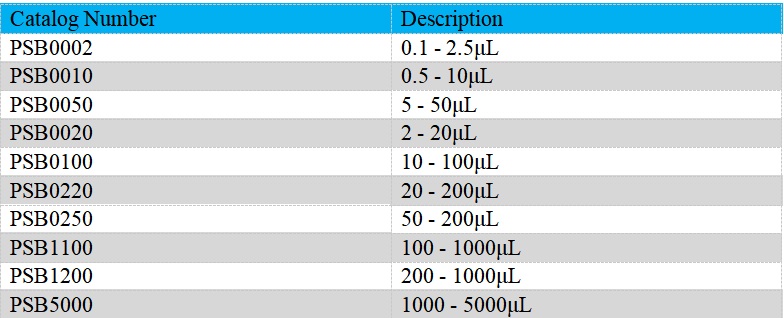
The Single-Channel Mechanical Pipettes (Semi-sterile) is adjustable, which could be used to dispense liquid precisely. Measuring volume of our Single-Channel Mechanical Pipettes (Semi-sterile) ranges from 0.1μL to 5mL. Products are produced based on ISO8655/DIN12650. It could be widely employed on endotoxin detection, etc..
2. Product Features
- Light weight, economic
- The pipettes cover volume range from 0.1μL to 5mL
- Semi-sterile
- Easy calibration and maintenance
Product detail pictures:

Related Product Guide:
We will make just about every exertion for being excellent and perfect, and speed up our actions for standing during the rank of worldwide top-grade and high-tech enterprises for 2022 wholesale price TAL assay - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo , The product will supply to all over the world, such as: Anguilla, Jamaica, Finland, If you are interested in any of our products and solutions or would like to discuss a custom order, remember to feel free to contact us. We are looking forward to forming successful business relationships with new clients around the world in the near future.
This supplier stick to the principle of "Quality first, Honesty as base", it is absolutely to be trust.