8 Year Exporter Define LAL Test - Eight-channel Mechanical Pipette – Bioendo
8 Year Exporter Define LAL Test - Eight-channel Mechanical Pipette – Bioendo Detail:
Eight-Channel Mechanical Pipettor
1. Product information
All multi-channel mechanical pipettor have been quality tested according to ISO8655-2:2002 with calibration certificate. The quality control involves gravimetric testing of each pipette with distilled water at 22℃. The multichannel mechanical pipettor is idea for the detectionof bacterial endotoxin lal endotoxin testing by kinetic turbidimetric andkinetic chromogenic method.
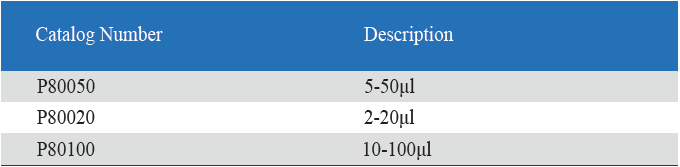
- Eight-Channel Mechanical Pipettor is available for standard 96-well plate
- Dispensing head rotates for optimum pipetting convenience
- Individual piston and tip cone assemblies allow easy repair and maintenance
- Compound material tip cone design allows visual seal verification
- Can be used with universal style pipette tips
-good for kinetic chromogenic, kinetic turbidimetric TAL orend-point chromogenic TAL endotoxin assay
Product detail pictures:

Related Product Guide:
With our leading technology at the same time as our spirit of innovation,mutual cooperation, benefits and growth, we're going to build a prosperous future together with your esteemed firm for 8 Year Exporter Define LAL Test - Eight-channel Mechanical Pipette – Bioendo , The product will supply to all over the world, such as: Algeria, El Salvador, Germany, We have top engineers in these industries and an efficient team in the research. What is more, now we have our own archives mouths and markets in China at low cost. Therefore, we can meet different inquiries from different clients. Remember to find our website to check more information from our merchandise.
Wide range, good quality, reasonable prices and good service, advanced equipment, excellent talents and continuously strengthened technology forces,a nice business partner.








