China wholesale LAL gel clot - Gel Clot Lyophilized Amebocyte Lysate Single Test in Ampoule – Bioendo
China wholesale LAL gel clot - Gel Clot Lyophilized Amebocyte Lysate Single Test in Ampoule – Bioendo Detail:
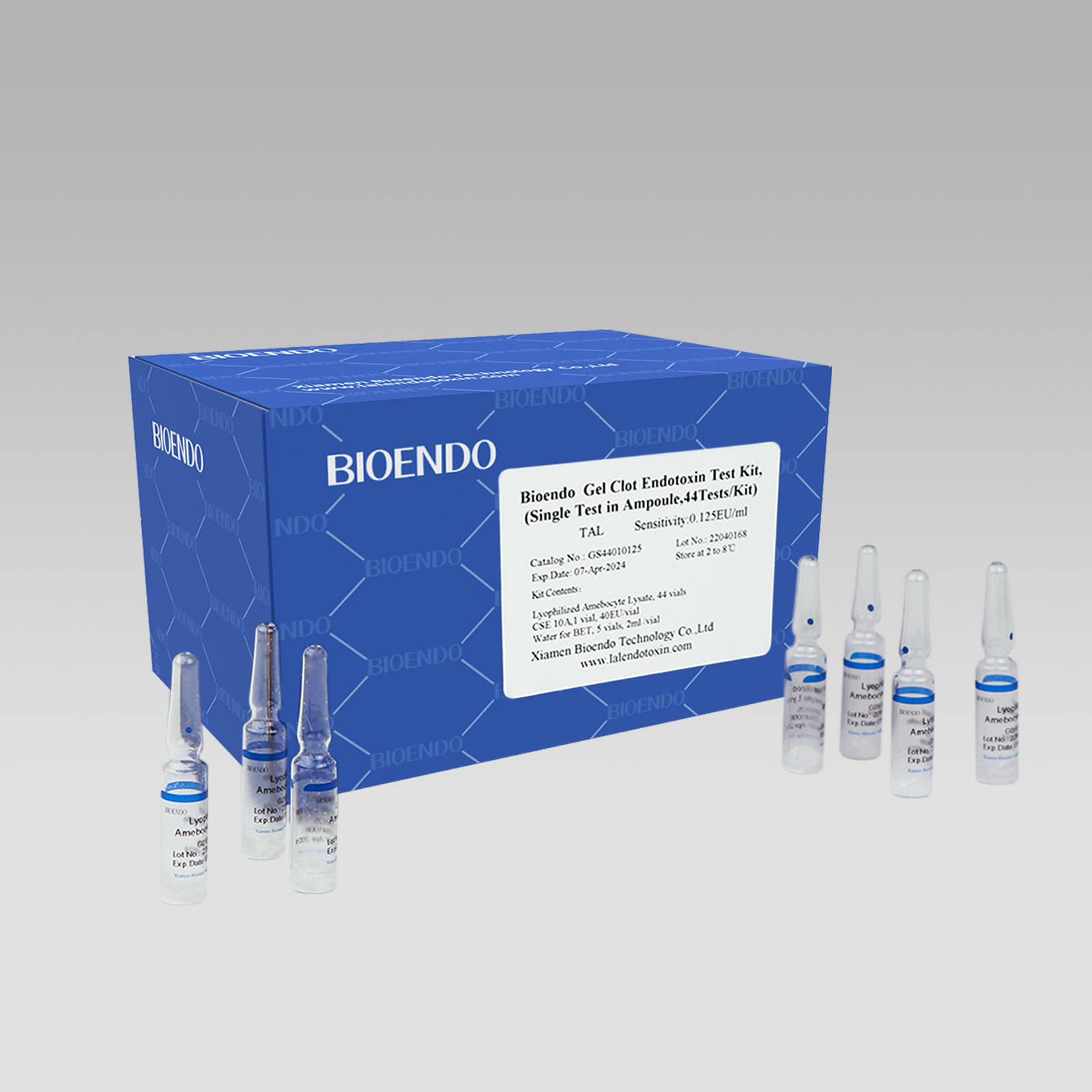
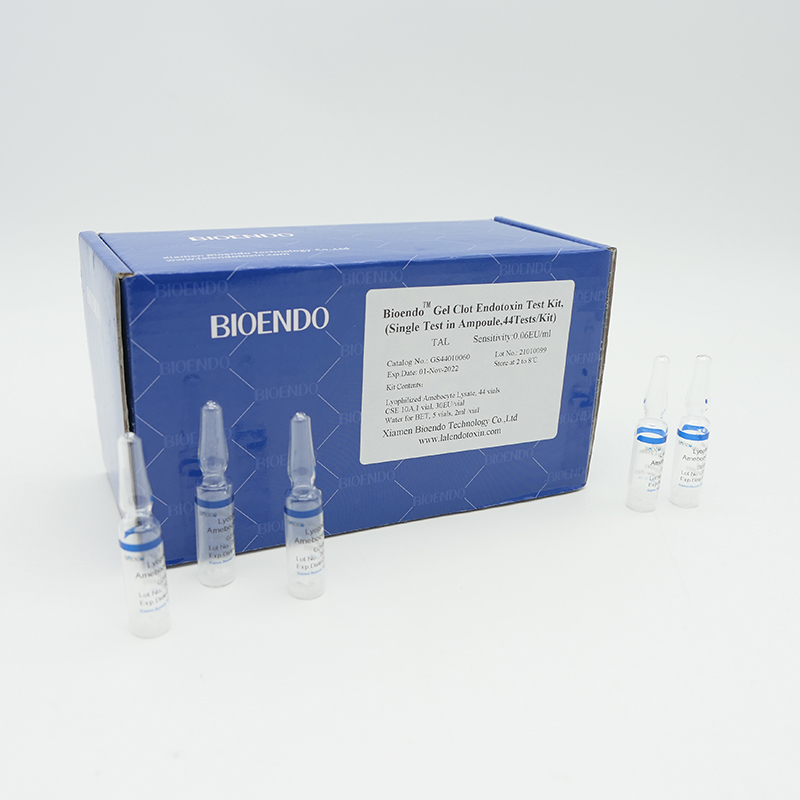
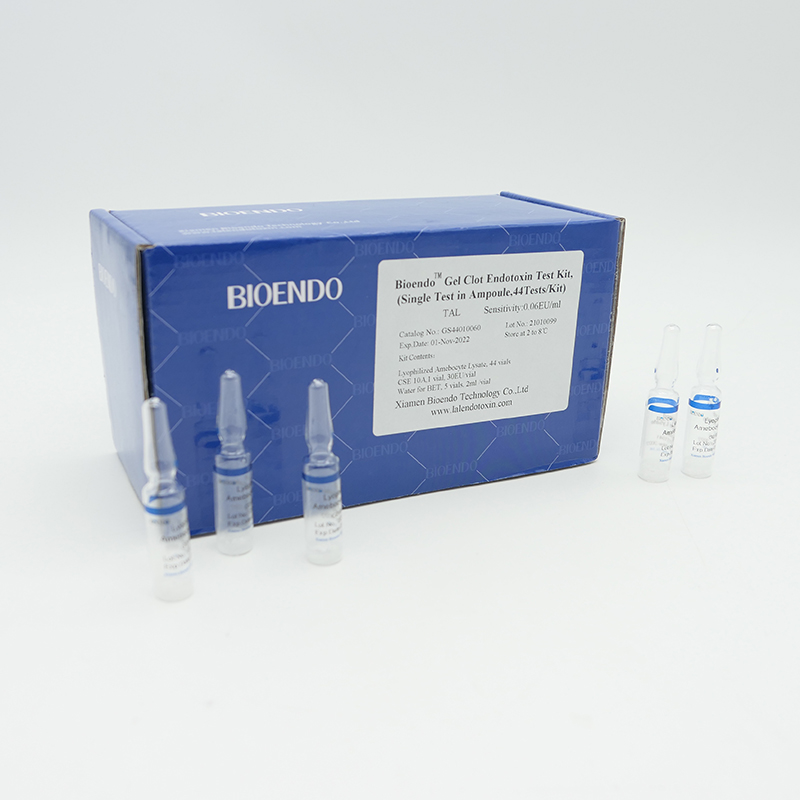
Gel Clot Lyophilized Amebocyte Lysate Single Test in Ampoule
1. Product Information
Gel Clot Lyophilized Amebocyte Lysate Single Test in ampoule Contain endotoxin-specific Amebocyte Lysate which includes beta-glucan inhibitor in the formulation and will not react to beta-glucan. For our Single Test in glass ampoules, you could add samples to the glass ampoules directly. This means you don’t need to reconstitute Amebocyte Lysate at first, and you will decide how many tests use each time to avoid waste. Endotoxin-free tubes for the dilution of Lyophilized CSE is necessary. Operation of endotoxin detection use Bioendo Gel Clot Lyophilized Amebocyte Lysate Single Test in ampoule conforms to the USP, the EP.
2. Product Parameter
Gel clot assay single test glass ampoule.
Sensitivities: 0.03EU/ml, 0.06EU/ml, 0.125EU/ml, 0.25 EU/ml
3.Product Features and Applications
Single step endotoxin detection,
Not requires sophisicated microplate reader, normal endotoxin assay incubator or water bath is able to use.
Suitable for end-product endotoxin testing before product released.
Product sensitivity standardized according to US Pharmacopoeia criterion and China Pharmacopoeia criterion.
Note:
Lyophilized Amebocyte Lysate (LAL) reagent manufactured by Bioendo is made from lysate of amebocytes (white blood cells) from the horseshoe crab.
|
Catalog No. |
Sensitivity EU/ml |
Description |
Kit Contents |
|
GS44010030 |
0.03 |
Bioendo Gel Clot Endotoxin Test Kit, (Single Test in Ampoule, 44Tests/Kit), |
44 Gel Clot Lyophilized Amebocyte Lysate; 1 CSE 10A; 5 Water for BET , 2ml/vial |
|
GS44010060 |
0.06 |
||
|
GS44010125 |
0.125 |
||
|
GS44010250 |
0.25 |
||
|
GS44010500 |
0.5 |
Product Condition
The sensitivity of Lyophilized Amebocyte Lysate and potency of Control Standard Endotoxin are assayed against USP Reference Standard Endotoxin. The Lyophilized Amebocyte Lysate reagent kits come with product instruction, Certificate of Analysis, MSDS.
Why the most selected gel clot assay kit GS44:
1. The most economical and commonly used lysate reagent for endotoxin detection.
2. Single test in a vial for one stepping reduce the risk of contamination, to provide the best convenience for the LAL test.
3. Gel clot LAL assay single test no need sophisticated microplate reader, water bath or dry heat incubator as normal configuration in the LAL test endotoxin.
4. Saving endotoxin free tubes when using GS44 series to do bacterial endotoxin test in the procedure of endotoxin testing operation.
Product detail pictures:
Related Product Guide:
We are experienced manufacturer. Wining the majority from the crucial certifications of its market for China wholesale LAL gel clot - Gel Clot Lyophilized Amebocyte Lysate Single Test in Ampoule – Bioendo , The product will supply to all over the world, such as: Brazil, Iran, Belarus, Our company has already had a lot of top factories and professional technology teams in China, offering the best products, techniques and services to worldwide customers. Honesty is our principle, professional operation is our work, service is our goal, and customers' satisfaction is our future!
It is not easy to find such a professional and responsible provider in today's time. Hope that we can maintain long-term cooperation.