Chinese Professional LAL testing medical devices - Endotoxin Assay Kit for Human Plasma – Bioendo
Chinese Professional LAL testing medical devices - Endotoxin Assay Kit for Human Plasma – Bioendo Detail:
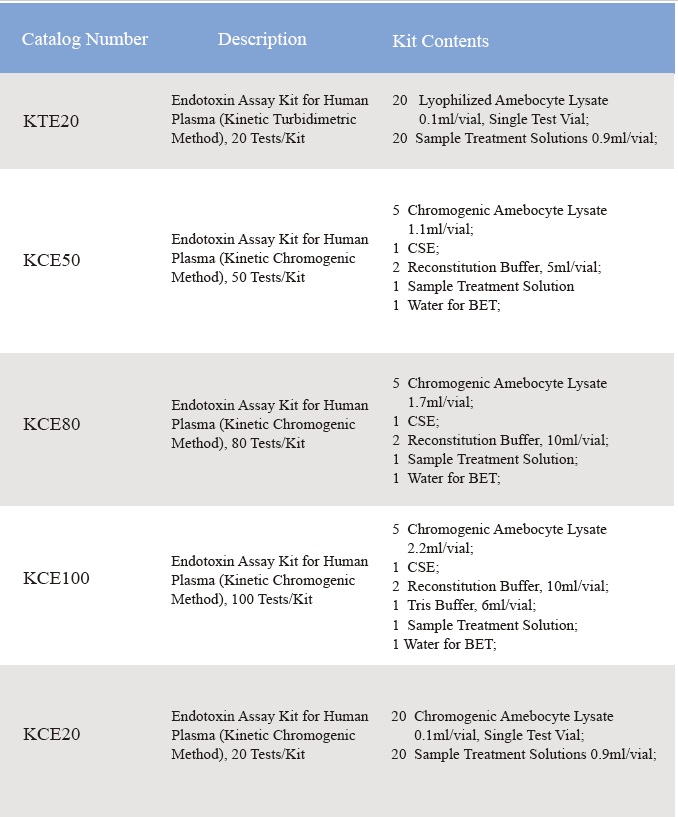
Endotoxin Assay Kit for Human Plasma
1. Product Information
CFDA cleared Clinical diagnostic Endotoxin assay kit quantifies endotoxin level inhuman plasma. Endotoxin is a major component of the cell wall of Gram Negative bacteria and is the most important microbial mediator of sepsis. Elevated levels of endotoxin can often induce fever, changes in white blood cell countsand, in some cases, cardiovascular shock. It is based on the factor Cpathway in limulus Polyphemus (horseshoe crab blood) test. With kinetic microplate reader and endotoxin assay software, Endotoxin assay kit detects endotoxin level in human plasma in less than one hour. The kit comes with the plasma pre-treatment reagent that eliminates the inhibition factors in plasma during the endotoxin assay.
2. Product Parameter
Assay range: 0.01-10 EU/ml
3. Product Feature and Application
Comes with plasma pretreatment solutions, eliminates the inhibition factors in the human plasma.
Note:
Lyophilized Amebocyte Lysate (LAL) reagent manufactured by Bioendo is made from amebocyte lysate derived blood of horseshoe crab.
The sensitivity of Lyophilized Amebocyte Lysate and potency of Control Standard Endotoxin are assayed against USP Reference Standard Endotoxin. The Lyophilized Amebocyte Lysate reagent kits come with product instruction, Certificate of Analysis, MSDS.
Product detail pictures:

Related Product Guide:
Our company promises all users of the first-class products and the most satisfying post-sale service. We warmly welcome our regular and new customers to join us for Chinese Professional LAL testing medical devices - Endotoxin Assay Kit for Human Plasma – Bioendo , The product will supply to all over the world, such as: Israel, Malta, Thailand, By integrating manufacturing with foreign trade sectors, we can present total customer solutions by guaranteeing the delivery of right merchandise to the right place at the right time, which is supported by our abundant experiences, powerful production capability, consistent quality, diversified products and the control of the industry trend as well as our maturity before and after sales services. We'd like to share our ideas with you and welcome your comments and questions.
Although we are a small company, we are also respected. Reliable quality, sincere service and good credit, we are honored to be able to work with you!