Chinese Professional LAL testing medical devices - Endotoxin Assay Kit for Human Plasma – Bioendo
Chinese Professional LAL testing medical devices - Endotoxin Assay Kit for Human Plasma – Bioendo Detail:
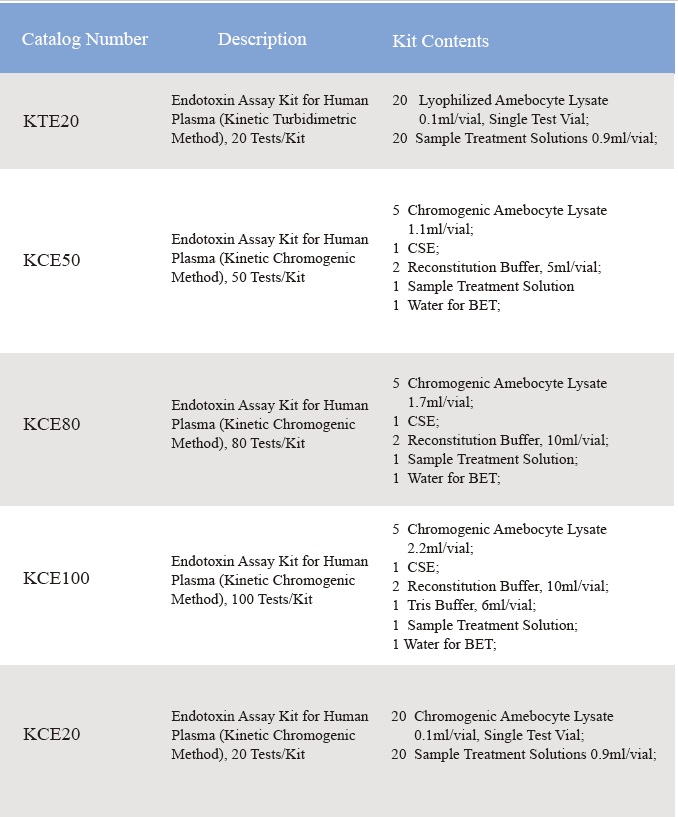
Endotoxin Assay Kit for Human Plasma
1. Product Information
CFDA cleared Clinical diagnostic Endotoxin assay kit quantifies endotoxin level inhuman plasma. Endotoxin is a major component of the cell wall of Gram Negative bacteria and is the most important microbial mediator of sepsis. Elevated levels of endotoxin can often induce fever, changes in white blood cell countsand, in some cases, cardiovascular shock. It is based on the factor Cpathway in limulus Polyphemus (horseshoe crab blood) test. With kinetic microplate reader and endotoxin assay software, Endotoxin assay kit detects endotoxin level in human plasma in less than one hour. The kit comes with the plasma pre-treatment reagent that eliminates the inhibition factors in plasma during the endotoxin assay.
2. Product Parameter
Assay range: 0.01-10 EU/ml
3. Product Feature and Application
Comes with plasma pretreatment solutions, eliminates the inhibition factors in the human plasma.
Note:
Lyophilized Amebocyte Lysate (LAL) reagent manufactured by Bioendo is made from amebocyte lysate derived blood of horseshoe crab.
The sensitivity of Lyophilized Amebocyte Lysate and potency of Control Standard Endotoxin are assayed against USP Reference Standard Endotoxin. The Lyophilized Amebocyte Lysate reagent kits come with product instruction, Certificate of Analysis, MSDS.
Product detail pictures:

Related Product Guide:
Good quality comes 1st; assistance is foremost; business enterprise is cooperation" is our business enterprise philosophy which is regularly observed and pursued by our company for Chinese Professional LAL testing medical devices - Endotoxin Assay Kit for Human Plasma – Bioendo , The product will supply to all over the world, such as: Pakistan, kazan, Macedonia, With well educated, innovative and energetic staff, we are responsible for all elements of research, design, manufacture, sale and distribution. By studying and developing new techniques, we are not only following but also leading fashion industry. We listen attentively to the feedback from our customers and provide instant replies. You will instantly feel our professional and attentive service.
As an international trading company, we have numerous partners, but about your company, I just want to say, you are really good, wide range, good quality, reasonable prices, warm and thoughtful service, advanced technology and equipment and workers have professional training, feedback and product update is timely, in short, this is a very pleasant cooperation, and we look forward to the next cooperation!