Chinese wholesale Chromogenic endotoxin assay - Bioendo KC Endotoxin Test Kit (Kinetic Chromogenic Assay) – Bioendo
Chinese wholesale Chromogenic endotoxin assay - Bioendo KC Endotoxin Test Kit (Kinetic Chromogenic Assay) – Bioendo Detail:
Bioendo KC Endotoxin Test Kit (Kinetic Chromogenic Assay)
1. Product Information
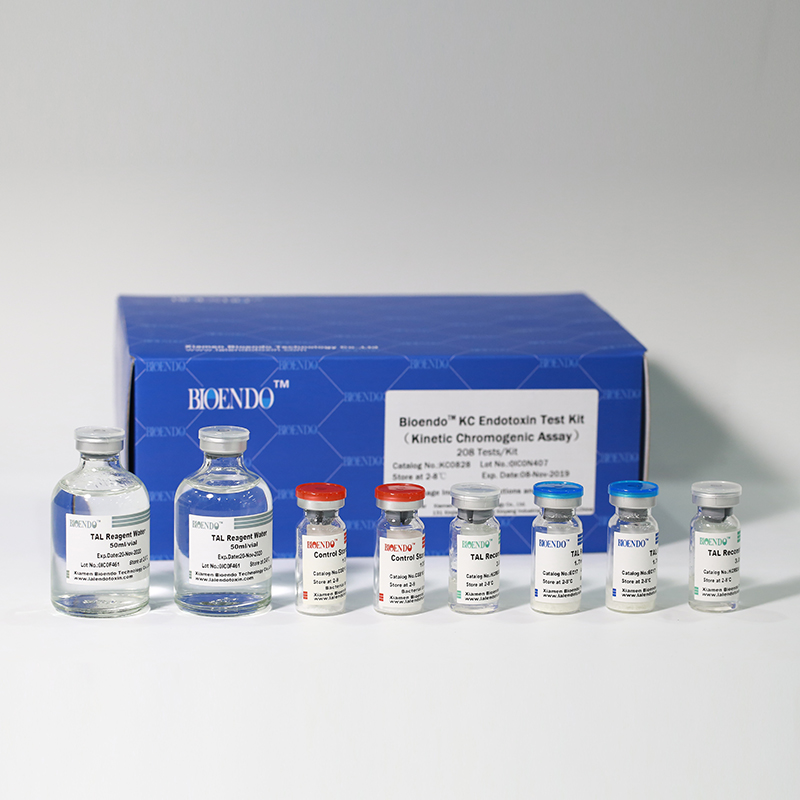
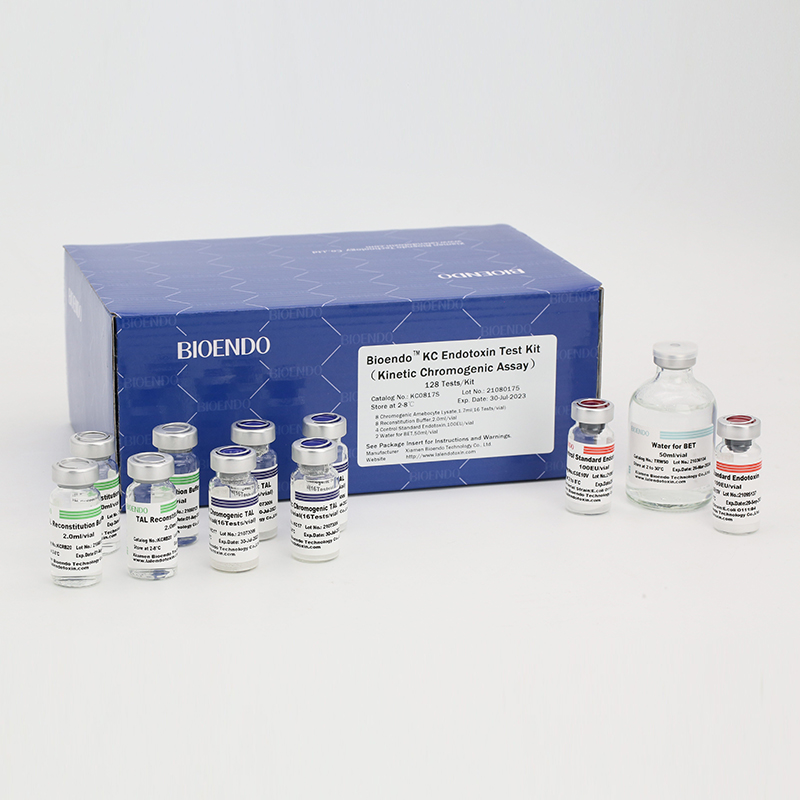
In Bioendo KC Endotoxin Test Kit, Amebocyte Lysate is co-lyophilized with chromogenic substrate. Therefore, bacterial endotoxin could be quantified based on the chromogenic reaction. The assay is strong resistance to interference, and has advantages of kinetic turbidimetric and end-point chromogenic method. Bioendo Endotoxin Test Kit contains Chromogenic Amebocyte Lysate, Reconstitution Buffer, CSE, Water for BET. Endotoxin detection with Kinetic Chromogenic method requires a kinetic incubating microplate reader such as ELx808IULALXH.
2. Product Parameter
Assay Range: 0.005 – 50EU/ml; 0.001 – 10EU/ml
|
Catalog No. |
Description |
Kit Contents |
Sensitivity EU/ml |
|
KC5028 |
Bioendo™ KC Endotoxin Test Kit (Kinetic Chromogenic Assay), 1300 Tests/Kit |
50 Chromogenic Amebocyte Lysate, 2.8ml (26 Tests/Vial); 50 Reconstitution Buffer, 3.0ml/vial; 10CSE; |
0.005-5EU/ml |
|
KC5028S |
0.001-10EU/ml |
||
|
KC0828 |
Bioendo™ KC Endotoxin Test Kit (Kinetic Chromogenic Assay), 208 Tests/Kit |
8 Chromogenic Amebocyte Lysate, 2.8ml (26 Tests/Vial); 8 Reconstitution Buffer, 3.0ml/vial; 4 CSE; 2 Water for BET, 50ml/vial; |
0.005-5EU/ml |
|
KC0828S |
0.001-10EU/ml |
||
|
KC5017 |
Bioendo™ KC Endotoxin Test Kit (Kinetic Chromogenic Assay), 800 Tests/Kit |
50 Chromogenic Amebocyte Lysate, 1.7ml (16 Tests/Vial); 50 Reconstitution Buffer, 2.0ml/vial; 10CSE; |
0.005-5 EU/ml |
|
KC5017S |
0.001-10 EU/m |
||
|
KC0817 |
Bioendo™ KC Endotoxin Test Kit (Kinetic Chromogenic Assay), 128 Tests/kit |
8 Kinetic Chromogenic Amebocyte Lysate, 1.7ml (16 Tests/vial); 8 Reconstitution Buffer, 2.0ml/vial; 4 CSE; 2 Water for BET, 50ml/vial; |
0.005-5 EU/ml |
|
KC0817S |
0.001-10 EU/ml |
3. Product Feature and Application
BioendoTM KC Endotoxin Test Kit (Kinetic Chromogenic Assay) features strong resistance to interference, and has advantages of kinetic turbidimetric and end-point chromogenic method. It is especially suitable for endotoxin detection of biological samples like vaccine, antibody, protein, nucleic acid, etc.
Note:
Lyophilized Amebocyte Lysate reagent manufactured by Bioendo is made from amebocyte lysate from the horseshoe crab (Tachypleus tridentatus).
Product Condition:
The sensitivity of Lyophilized Amebocyte Lysate and potency of Control Standard Endotoxin are assayed against USP Reference Standard Endotoxin. The Lyophilized Amebocyte Lysate reagent kits come with product instruction, Certificate of Analysis.
The kinetic chromogenic endotoxin test kit have to choose the microplate reader with 405nm filters.
Product detail pictures:



Related Product Guide:
We believe that long time period partnership is a result of top of the range, value added services, rich expertise and personal contact for Chinese wholesale Chromogenic endotoxin assay - Bioendo KC Endotoxin Test Kit (Kinetic Chromogenic Assay) – Bioendo , The product will supply to all over the world, such as: Paris, Johannesburg, Algeria, We have developed large markets in many countries, such as Europe and the United States, Eastern Europe and Eastern Asia. Meanwhile with the powerful predominance in persons with ability, strict production management and business concept.we constantly carry on self-innovation, technological innovation, managing innovation and business concept innovation. To follow the world markets fashion, new products are kept on researching and providing to guarantee our competitive advantage in styles, quality, price and service.
The factory can meet continuously developing economic and market needs, so that their products are widely recognized and trusted, and that's why we chose this company.








