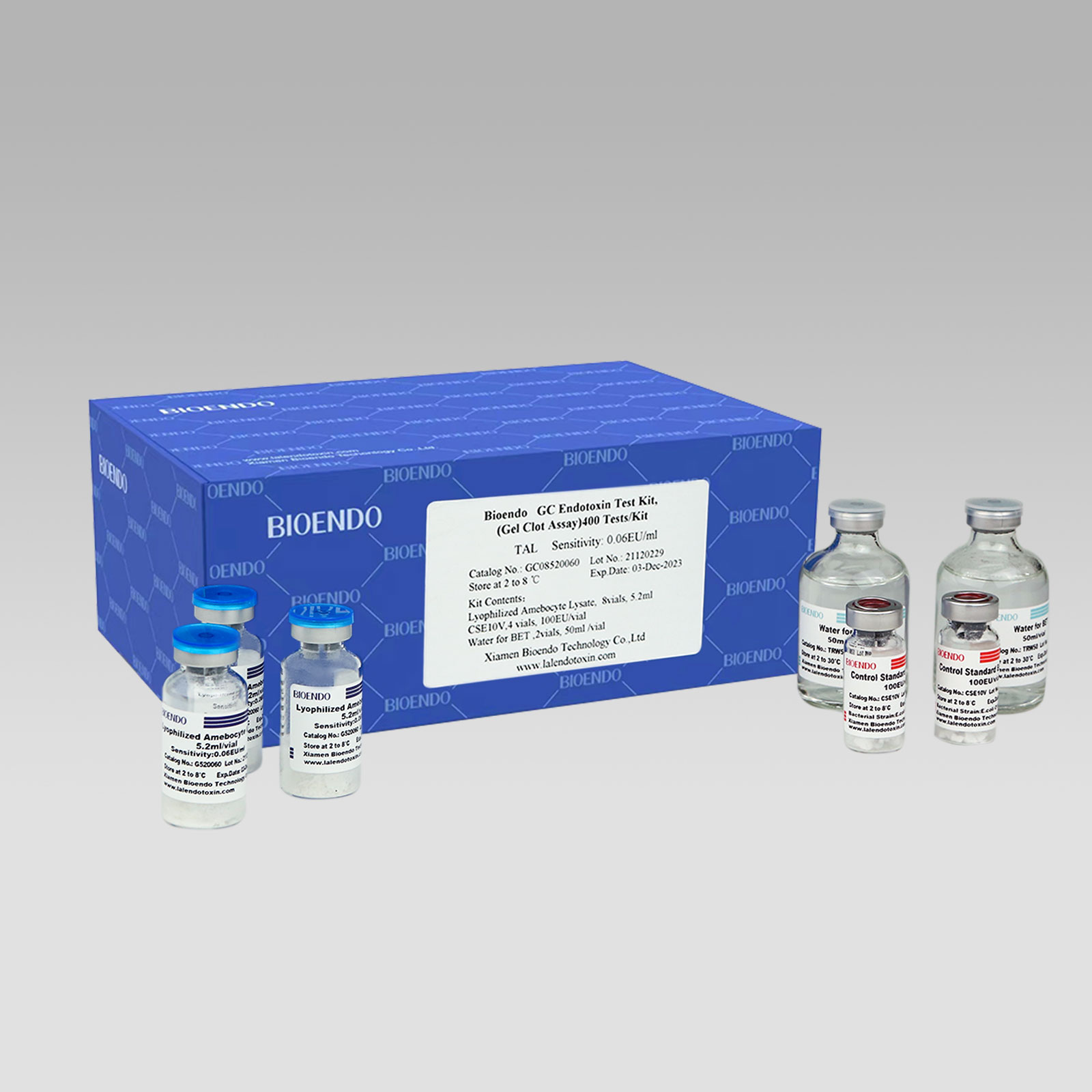
Bioendo GC Endotoxin Test Kit (Gel Clot Assay)
Bioendo GC Endotoxin Test Kit (Gel Clot Assay)
1. Product Information
Bioendo GC Endotoxin Test Kit (Gel Clot Assay) contains 128 Tests/Kit, 400Tests/Kit, 1600Tests/Kit, and 4500 Tests/Kit. And sensitivity of Amebocyte Lysate included by the Kit is 0.03 EU/ml, 0.06 EU/ml, 0.125 EU/ml, 0.25 EU/ml, 0.5 EU/ml. The Kit contains Amebocyte Lysate, CSE and Water for BET. Four kinds of configuration of gel-clot endotoxin assay kit to satisfied the demand. This means you could choose different configuration kits according to the requirement.
2. Product Parameter
Sensitivities: 0.03EU/ml, 0.06EU/ml, 0.125EU/ml, 0.25EU/ml, 0.5 EU/ml
3.Product Features and Applications
Full range of sensitivities to meet the demand when operate of LAL endotoxin assay.
No need special and sophisticated instrument, dry heat incuabtor is very smart and sitable. Endotoxin detection with Bioendo GC Endotoxin Test Kit (Gel Clot Assay) to meet your mass samples to be tested endotoxins.
It is the best choice when you need to do a variety of LAL test endotoxin with gel clot method GC TAL test kits.
Note:
Lyophilized Amebocyte Lysate (LAL reagent / LAL reagent) manufactured by Bioendo is made from amebocyte lysate derived blood of horseshoe crab.
|
Catalog No. |
Sensitivity (EU/ml) |
Description |
Kit Contents |
|
GC90520030 |
0.03 |
Bioendo™ GC Endotoxin Test Kit (Gel Clot Assay) 4500 Tests/Kit |
90 Gel Clot Lyophilized Amebocyte Lysate, 5.2ml (50 Tests/Vial); 20 CSE10V; |
|
GC90520060 |
0.06 |
||
|
GC90520125 |
0.125 |
||
|
GC90520250 |
0.25 |
||
|
GC90520500 |
0.5 |
||
|
GC08520030 |
0.03 |
Bioendo™ GC Endotoxin Test Kit (Gel Clot Assay) 400 Tests/Kit |
8 Gel Clot Lyophilized Amebocyte Lysate, 5.2ml (50 Tests/Vial); 4 CSE10V; 2 Water for BET, 50ml/vial; |
|
GC08520060 |
0.06 |
||
|
GC08520125 |
0.125 |
||
|
GC08520250 |
0.25 |
||
|
GC08520500 |
0.5 |
||
|
GC100170030 |
0.03 |
Bioendo™ GC Endotoxin Test Kit (Gel Clot Assay) 1600 Tests/Kit |
100 Gel Clot Lyophilized Amebocyte Lysate, 1.7ml (16 Tests/Vial); 10 CSE10V; |
|
GC100170060 |
0.06 |
||
|
GC100170125 |
0.125 |
||
|
GC100170250 |
0.25 |
||
|
GC100170500 |
0.5 |
||
|
GC08170030 |
0.03 |
Bioendo™ GC Endotoxin Test Kit (Gel Clot Assay) 128 Tests/Kit |
8 Gel Clot Lyophilized Amebocyte Lysate, 1.7ml (16 Tests/Vial); 4 CSE10V; 2 Water for BET, 50ml/vial; |
|
GC08170060 |
0.06 |
||
|
GC08170125 |
0.125 |
||
|
GC08170250 |
0.25 |
||
|
GC08170500 |
0.5 |
Product condition:
LAL assay endotoxin Test kit’s sensitivity and the Control Standard Endotoxin potency are assayed against USP Reference Standard Endotoxin. The Limulus Amebocyte Lysate kits come with product instruction, Certificate of Analysis.
Related products in the LAL test for pyrogens:
Water for Bacterial Endotoxins Test (BET), typical TRW50 or TRW100, Endotoxin-free level is very necessary in the LAL assay endotoxin‘s operation.
Endotoxin free glass tube ( dilution tube ), T1310018 and T107540, dilution tube and reaction tube its size is not the same in the endotoxin testing.
Pyrogen free tips, Recommend PT25096 or PT100096, normal steile tips are not available in operation of LAL test.
Pipettor, Recommend PSB0220
Test Tube Rack
Incubation Instrument (Water Bath or Dry Heat Incubator ), to recommend Bioendo Dry Heat Incubator TAL-M2 is 60 holes one modular.
Vortex Mixter, Recommend VXH.
Control Standard Endotoxin, CSE10V.