Factory Cheap Hot endotoxin removal kit – EtEraser™ SE Endotoxin Removal Kit – Bioendo
Factory Cheap Hot endotoxin removal kit – EtEraser™ SE Endotoxin Removal Kit – Bioendo Detail:
EtEraser™ SE Endotoxin Removal Kit
1. Product information
Lipopolysaccharide (LPS) is a bacterial endotoxin and a majorconstituent of the cell walls of gramnegative bacteria. Recombinant proteinfrom E.coli usually contains high level of endotoxins. The removal of theseendotoxins is highly necessary for downstream processes.
EtEraser SE Endotoxin Removal kit are designedto remove endotoxin contamination from aqueous solutions. The kits containendotoxin removal resin binds to reduce endotoxin levels in protein samples by≥99% in less than 2 hours.
The kit includes a pre-packed endotoxin removal column1.5 ml, Equilibration Buffer, Regeneration Buffer and pyrogen-freecollection tube and tips. The column has a high binding capacity of > 2,000, 000 EU / ml. This product may be reused up to five times if properlyregenerated. The affinity resin is available in slurry and could be up-scalingin biopharmaceutical process.
EtEraser SE Endotoxin Removal kit utilizes food grade modified ε-poly-L-lysine ashigh affinity ligand for endotoxins. The resin is agarose beads which is verystable and has no cytotoxic effect to human body. After purification, therewill no toxic residual remained in the samples. This endotoxin removal kit canbe used in protein drugs, vaccines, antibodies, DNA/RNA, polysaccharide andother biological samples.
2. Product features
• high stability — does not affect the activity of most of thebiological sample
• high bonding capacity — >2000000 EU/ml
• non-toxic —–> Non-toxic food grade ligands, no cytotoxiceffects poly lysine
• high protein recovery — >95% protein recovery for proteinsamples
• high removal efficiency — remove >99% endotoxin,endotoxin level in the sample can be less than 0.1 EU/ml after endotoxinremoving process
• wide application range — can be used in endotoxin removalfor proteins, peptides, antibodies, vaccines, polysaccharide and otherbiological samples
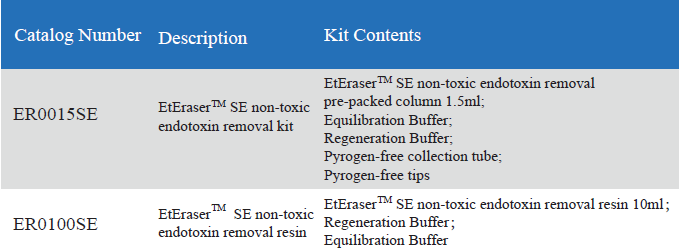

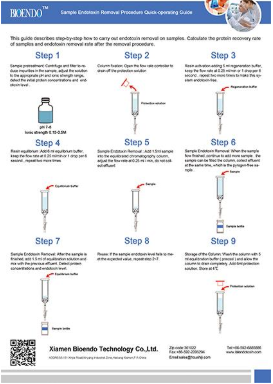
Product detail pictures:

Related Product Guide:
Our eternal pursuits are the attitude of "regard the market, regard the custom, regard the science" as well as theory of "quality the basic, have faith in the initial and administration the advanced" for Factory Cheap Hot endotoxin removal kit – EtEraser™ SE Endotoxin Removal Kit – Bioendo , The product will supply to all over the world, such as: Romania, Jordan, Croatia, With the development and enlargement of mass clients abroad, now we've set up cooperative relationships with many major brands. We've our own factory and also have many reliable and well-cooperated factories in the field. Adhering to the "quality first, customer first, We are provideing high-quality, low-cost items and first-class service to customers. We sincerely hope to establish business relationship with customers from all over the world on the basis of quality, mutually benefit. We welcome OEM projects and designs.
Wide range, good quality, reasonable prices and good service, advanced equipment, excellent talents and continuously strengthened technology forces,a nice business partner.








