Factory Free sample Coagulation Gel - Rapid Gel Clot 10 Samples Kit – Bioendo
Factory Free sample Coagulation Gel - Rapid Gel Clot 10 Samples Kit – Bioendo Detail:
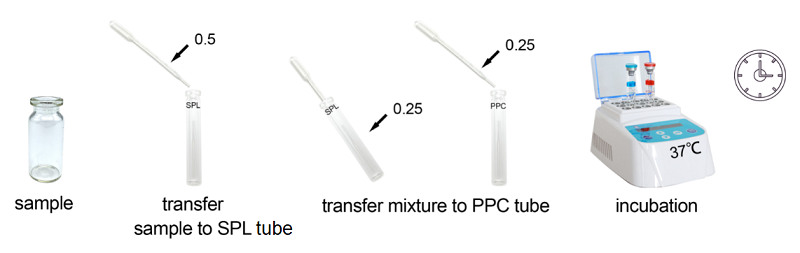
Bioendo Rapid Gel Clot Endotoxin Assay Kit is designed to rapidly quantify endotoxin in water or dialysate. Generally, RG kit’s result could be gained within 30 minutes. Under the guidance of detecting endotoxin in water or dialysate quickly, endotoxin detection with Bioendo Rapid Gel Clot Endotoxin Assay Kit does not need the multi steps’ dilution of Control Standard Endotoxin and test samples. Operation procedures are very convenient, additional experimental equipment are required. It is a convenient and rapid way to detect endotoxin in especial suitable for water or dialysate.
2. Product Parameter
Sensitivity Range: 0.03EU/ml, 0.06EU/ml, 0.125EU/ml, 0.25EU/ml, 0.5EU/ml
10 sample tests in the kit.
Assay time: less than 30 minutes
3. Product Application
Bioendo Rapid Gel Clot Endotoxin Assay Kit is designed to rapidly quantify endotoxin in water or dialysate as well as do quickly endotoxin detection in life science research.
Note:
Lyophilized Amebocyte Lysate (LAL) reagent manufactured by Bioendo is made from amebocyte lysate derived blood of horseshoe crab.
|
Catalog No. |
Description |
Kit Contents |
Sensitivity EU/ml |
Reaction Time minutes |
|
RG10025003 |
BioendoTM Rapid Gel Clot Endotoxin Assay Kit, 10 Samples Kit |
10 SPL Tubes; 10 PPC Tubes; 10 Endotoxin-free Sample Bottles; 10 Packs of (3pcs Transfer Pipettes) |
0.03 |
≤60 |
|
RG10025006 |
0.06 |
≤60 |
||
|
RG100250125 |
0.125 |
≤45 |
||
|
RG10025025 |
0.25 |
≤30 |
||
|
RG10025050 |
0.5 |
≤30 |
Product detail pictures:



Related Product Guide:
With our leading technology at the same time as our spirit of innovation,mutual cooperation, benefits and growth, we're going to build a prosperous future together with your esteemed firm for Factory Free sample Coagulation Gel - Rapid Gel Clot 10 Samples Kit – Bioendo , The product will supply to all over the world, such as: Spain, Nicaragua, Lebanon, Being the top solutions of our factory, our solutions series have been tested and won us experienced authority certifications. For additional parameters and item list details, be sure to click the button to acquire additional nformation.
Timely delivery, strict implementation of the contract provisions of the goods, encountered special circumstances, but also actively cooperate, a trustworthy company!







