factory low price Bacterial Endotoxin Test Calculation - Control Standard Endotoxin (CSE) – Bioendo
factory low price Bacterial Endotoxin Test Calculation - Control Standard Endotoxin (CSE) – Bioendo Detail:
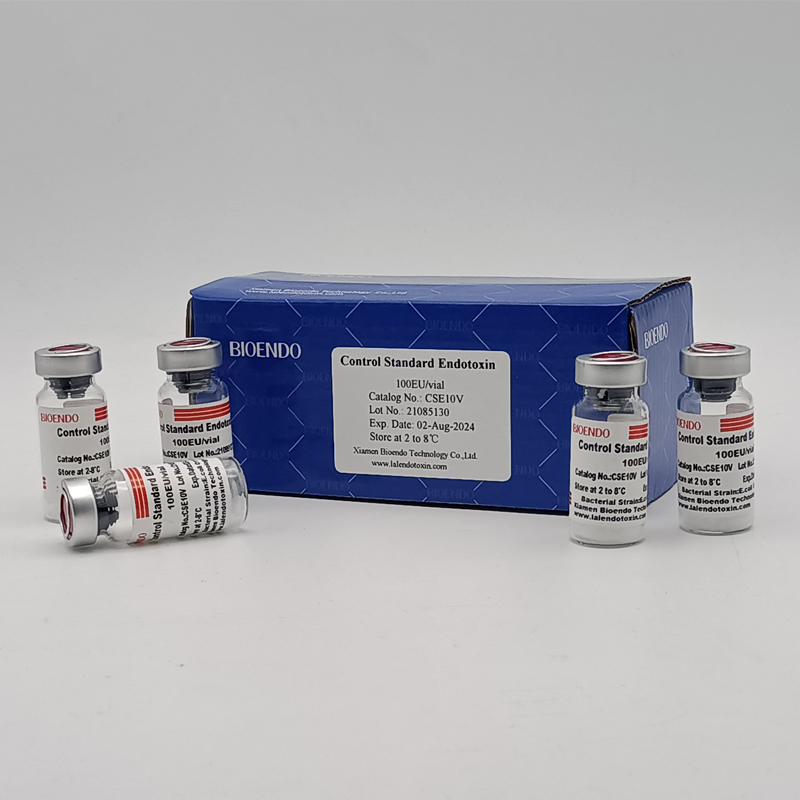
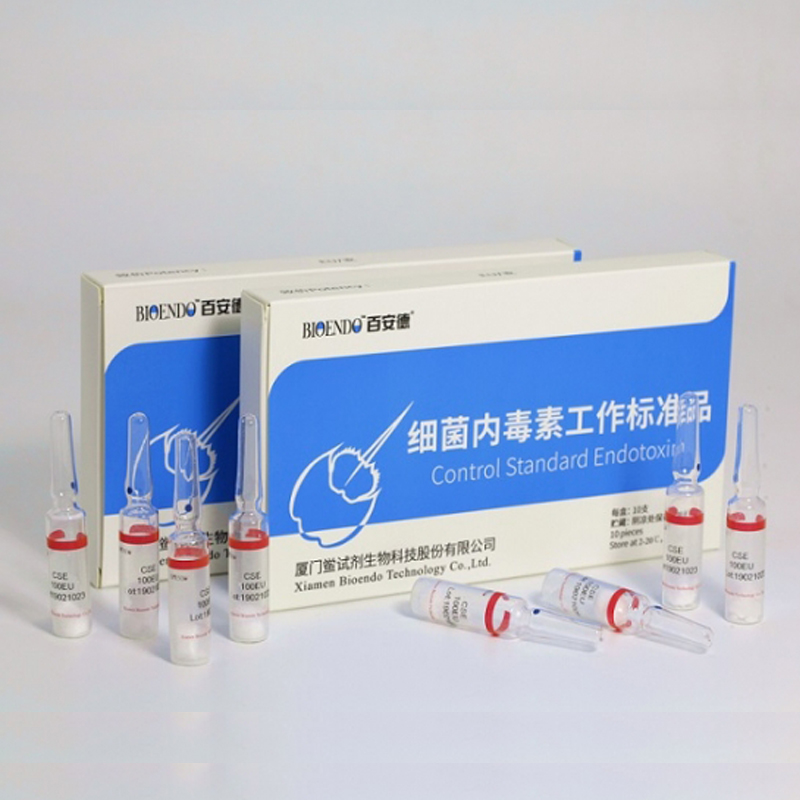
Control Standard Endotoxin (CSE)
1. Product Information
Control Standard Endotoxin (CSE) is extracted from E.coli O111:B4. CSE is an economic alternative to Reference Standard Endotoxin (RSE) in constructing standard curves, validating product and preparing controls in Lyophilized Amebocyte Lysate test. The labeled potency of CSE endotoxinE.coli standard is referenced against RSE. The Control Standard Endotoxin could be used with gel clot assay, kinetic turbidimetric assay or kinetic chromogenic assay as the endotoxin testing standards. The Certificate of Analysis will show the matched Lyophilized Amebocyte Lysate reagent lots.
2. Product Parameter
| Catalog Number | Potency (EU/vial) | Package |
| CSE10V | 100 to 999 EU | seal in glass vial, 10vials/pack |
| CSE100V | 1 to 199 EU | seal in glass vial, 10vials/pack |
| CSE10A | 1 to 99 EU | seal in glass ampoule, 10vials/pack |
3. Product Feature and Application
Bioendo CSE was labeled by the potency and matched to Lyophilized Amebocyte Lysate reagent lots. Users do not need to do the CSE/RSE ratio assay. Low potency control standard endotoxin is available to avoid lots of steps of dilution to provide convenience for end users.
Product Condition:
Control Standard Endotoxin (CSE), extracted from E.coli O111:B4, is an economic alternative to Reference Standard Endotoxin (RSE) in constructing standard curves, validating product and preparing controls in endotoxin test. The potency of CSE is referenced against USP Reference Standard Endotoxin, and labeled in the Certificate of Analysis.
Endotoxin test assay: Lysate reagent and CSE lot number have to be matched.
Pyrogen free tip box
Endotoxin free tubes
Product detail pictures:


Related Product Guide:
With our loaded practical experience and thoughtful solutions, we now have been identified for a trusted provider for numerous intercontinental consumers for factory low price Bacterial Endotoxin Test Calculation - Control Standard Endotoxin (CSE) – Bioendo , The product will supply to all over the world, such as: Dubai, Muscat, Slovenia, As a way to use the resource on the expanding info in international trade, we welcome prospects from everywhere on the web and offline. In spite on the high quality items we offer, effective and satisfying consultation service is supplied by our qualified after-sale service group. Item lists and detailed parameters and any other info weil be sent to you timely for the inquiries. So please make contact with us by sending us emails or call us when you've got any questions about our organization. ou could also get our address information from our site and come to our enterprise. We get a field survey of our merchandise. We are confident that we'll share mutual accomplishment and create solid co-operation relations with our companions within this market place. We're seeking forward for your inquiries.
The company comply with the contract strict, a very reputable manufacturers, worthy a long-term cooperation.





