Factory source Advantage Of LAL Test - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo
Factory source Advantage Of LAL Test - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo Detail:
Single-Channel Mechanical Pipettes (Semi-sterile)
1. Product Information
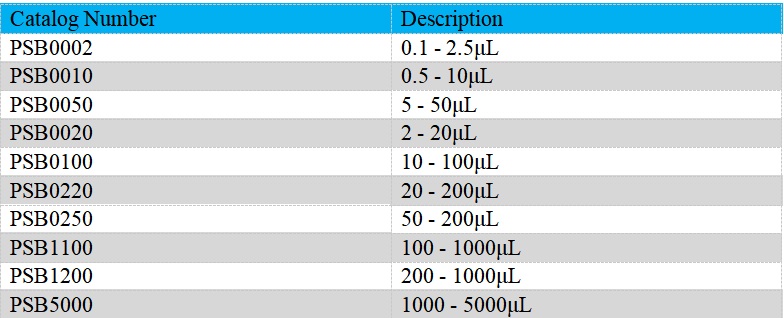
The Single-Channel Mechanical Pipettes (Semi-sterile) is adjustable, which could be used to dispense liquid precisely. Measuring volume of our Single-Channel Mechanical Pipettes (Semi-sterile) ranges from 0.1μL to 5mL. Products are produced based on ISO8655/DIN12650. It could be widely employed on endotoxin detection, etc..
2. Product Features
- Light weight, economic
- The pipettes cover volume range from 0.1μL to 5mL
- Semi-sterile
- Easy calibration and maintenance
Product detail pictures:

Related Product Guide:
Sticking to the principle of "Super Quality, Satisfactory service" ,We are striving to be a good business partner of you for Factory source Advantage Of LAL Test - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo , The product will supply to all over the world, such as: Chile, Mexico, Egypt, Item have passed by means of the national qualified certification and been well received in our main industry. Our expert engineering team will often be ready to serve you for consultation and feedback. We are able to also deliver you with cost-free samples to meet your specifications. Ideal efforts will probably be produced to provide you the most beneficial service and solutions. Should you be interested in our company and solutions, please make contact with us by sending us emails or call us straight away. To be able to know our solutions and enterprise. ar more, you'll be able to come to our factory to see it. We'll constantly welcome guests from all over the world to our firm. o build business enterprise. elations with us. Please feel absolutely free to speak to us for organization. nd we believe we will share the best trading practical experience with all our merchants.
Problems can be quickly and effectively resolved, it is worth to be trust and working together.







