Factory source Advantage Of LAL Test - Single-Channel Mechanical Pipettor – Bioendo
Factory source Advantage Of LAL Test - Single-Channel Mechanical Pipettor – Bioendo Detail:
Single-Channel Mechanical Pipettor
1. Product Information
Single channel mechanical pipette is ideal tool to support endotoxin detection with Lyophilized Amebocyte Lysate which covers gel-clot technique, kinetic turbidimetric technique, kinetic chromogenic technique, and end-point chromogenic technique. All pipettors are produced by following ISO8655 – 2:2002. The quality control involves gravimetric testing of each pipette with distilled water at 22℃.
2. Product Features:
- Light weight, economic, low force design
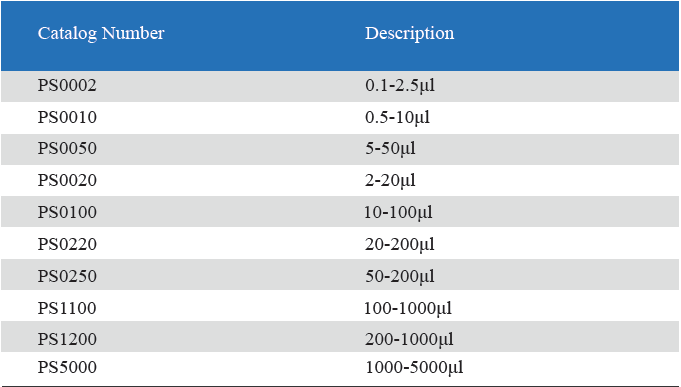
- Measuring volume range from 0.1μL to 5mL
- Easy to calibrate and maintain with tool supplied
- Design helps to avoid repetitive strain injuries
- Calibrated in accordance with ISO8655. Each pipettor supplied with individual test certificate
- The low part is available for autoclaving
Product detail pictures:

Related Product Guide:
Our goods are commonly recognized and reliable by consumers and may satisfy continually developing economic and social needs for Factory source Advantage Of LAL Test - Single-Channel Mechanical Pipettor – Bioendo , The product will supply to all over the world, such as: United States, Pretoria, Vietnam, Our business activities and processes are engineered to make sure our customers have access to widest range of products with the shortest supply time lines. This achievement is made possible by our highly skilled and experienced team. We look for people who want to grow with us around the globe and stand out from the crowd. We have people who embrace tomorrow, have vision, love stretching their minds and going far beyond what they thought was achievable.
As a veteran of this industry, we can say that the company can be a leader in the industry, select them is right.







