Factory wholesale Kinetic Turbidimetric LAL Assay - Bioendo KT Endotoxin Test Kit (Kinetic Turbidimetric Assay) – Bioendo
Factory wholesale Kinetic Turbidimetric LAL Assay - Bioendo KT Endotoxin Test Kit (Kinetic Turbidimetric Assay) – Bioendo Detail:
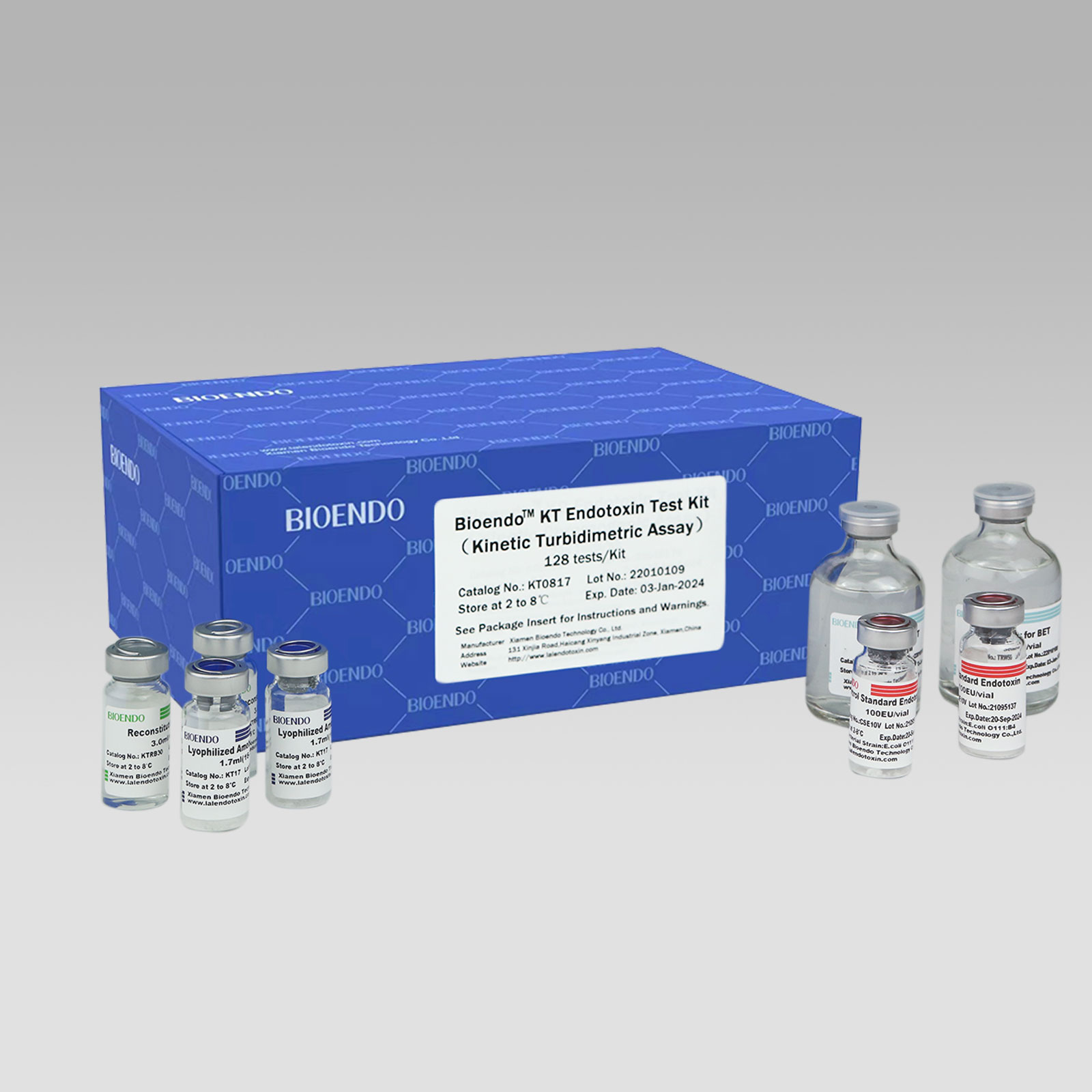
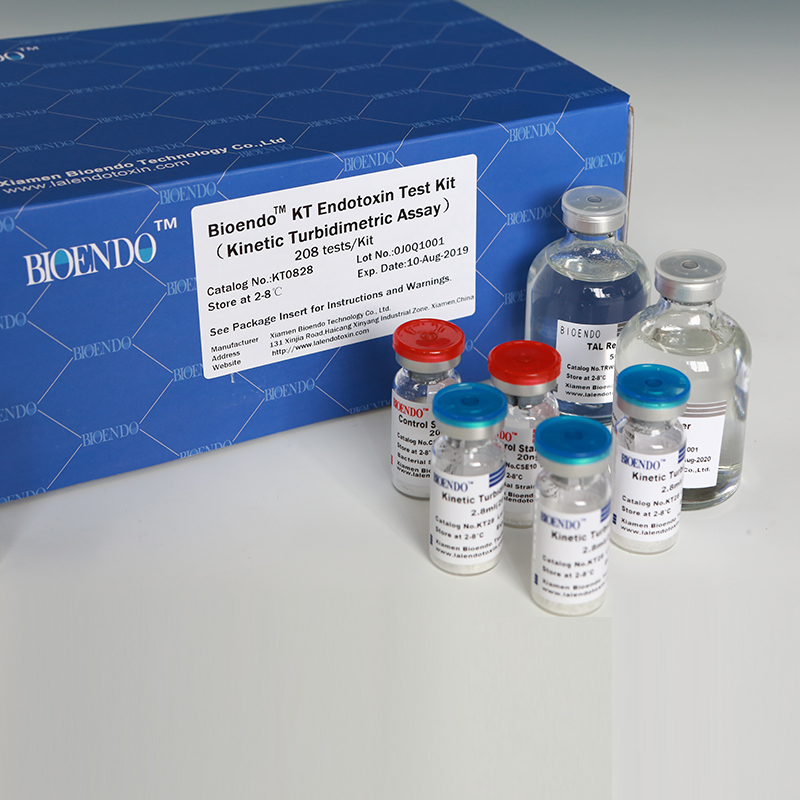
Bioendo KT Endotoxin Test Kit (Kinetic Turbidimetric Assay)
1. Product Introduction
Kinetic Turbidimetric Amebocyte Lysate Vial is developed based on the principle that the time needed to reach a certain absorbance increase (onset OD), i.e. onset time, is negatively correlated with the endotoxin concentration. Sensitivity could reach 0.005EU/ml, and the detection could reach four orders of magnitude. It is specially suitable for pharmaceuticals industry to monitor endotoxin concentration.
The kit contains Lyophilized Amebocyte Lysate, Control Standard Endotoxin, and Water for BET. KT Endotoxin Test Kit (Kinetic Turbidimetric Assay) require a kinetic microplate reader such as ELx808IULALXH or a kinetic tube reader. Kinetic software is also required for calculation of the endotoxin concentration.
2. Product Parameter
Assay Range: 0.005 – 5EU/ml; 0.01 – 10EU/ml
3. Product Application
End-product endotoxin (pyrogen) qualification, Waterfor injection endotoxin assay, raw material endotoxin testing or endotoxinlevel monitoring during manufacturing process for pharmaceutical companies ormedical devices manufacturers.
Note:
Lyophilized Amebocyte Lysate reagent manufactured by Bioendo is made from amebocyte lysate from the horseshoe crab (Tachypleus tridentatus).
|
Catalog No. |
Description |
Kit Contents |
Sensitivity EU/ml |
|
KT0817 |
Bioendo™ KT Endotoxin Test Kit (Kinetic Turbidimetric Assay), 128 tests/kit |
8 Lyophilized Amebocyte Lysate, 1.7ml (16 Tests/vial); 8 Reconstitution Buffer, 3.0ml/vial; 4 CSE; 2 Water for BET, 50ml/vial; |
0.01-10EU/ml |
|
KT0817S |
0.005-5EU/ml, 0.01-10EU/ml |
||
|
KT0852 |
Bioendo™ KT Endotoxin Test Kit (Kinetic Turbidimetric Assay), 400 Tests/Kit |
8 Lyophilized Amebocyte Lysate, 5.2ml (50 Tests/Vial); 8 Reconstitution Buffer, 6.0ml/vial; 4 CSE; 3 Water for BET, 50ml/vial; |
0.01-10EU/ml |
|
KT0852S |
0.005-5EU/ml, 0.01-10EU/ml |
||
|
KT5017 |
Bioendo™ KT Endotoxin Test Kit (Kinetic Turbidimetric Assay), 800 Tests/Kit |
50 Lyophilized Amebocyte Lysate, 1.7ml (16 Tests/vial); 50 Reconstitution Buffer, 3.0ml/vial; 10 CSE; |
0.01-10EU/ml |
|
KT5017S |
0.005-5EU/ml, 0.01-10EU/ml |
||
|
KT5052 |
Bioendo™ KT Endotoxin Test Kit (Kinetic Turbidimetric Assay), 2500 Tests/Kit |
50 Lyophilized Amebocyte Lysate,5.2ml (50 Tests/vial); 50 Reconstitution Buffer, 6.0ml/vial; 10 CSE; |
0.01-10EU/ml |
|
KT5052S |
0.005-5EU/ml, 0.01-10EU/ml |
Product Condition:
The potency of Lyophilized Amebocyte Lysate and Control Standard Endotoxin are assayed against USP Reference Standard Endotoxin. The Lyophilized Amebocyte Lysate reagent kits come with product instruction, Certificate of Analysis, MSDS.
Product detail pictures:


Related Product Guide:
With dependable high quality approach, great reputation and excellent customer support, the series of products and solutions produced by our firm are exported to lots of countries and regions for Factory wholesale Kinetic Turbidimetric LAL Assay - Bioendo KT Endotoxin Test Kit (Kinetic Turbidimetric Assay) – Bioendo , The product will supply to all over the world, such as: Spain, Japan, Slovakia, "Good quality and reasonable price" are our business principles. If you are interested in our products or have any questions, make sure you feel free to contact us. We hope to establish cooperative relationships with you in the near future.
This is a honest and trustworthy company, technology and equipment are very advanced and the prodduct is very adequate, there is no worry in the suppliment.








