Factory wholesale Kinetic Turbidimetric LAL Assay - Kinetic Turbidimetric Amebocyte Lysate Vial – Bioendo
Factory wholesale Kinetic Turbidimetric LAL Assay - Kinetic Turbidimetric Amebocyte Lysate Vial – Bioendo Detail:
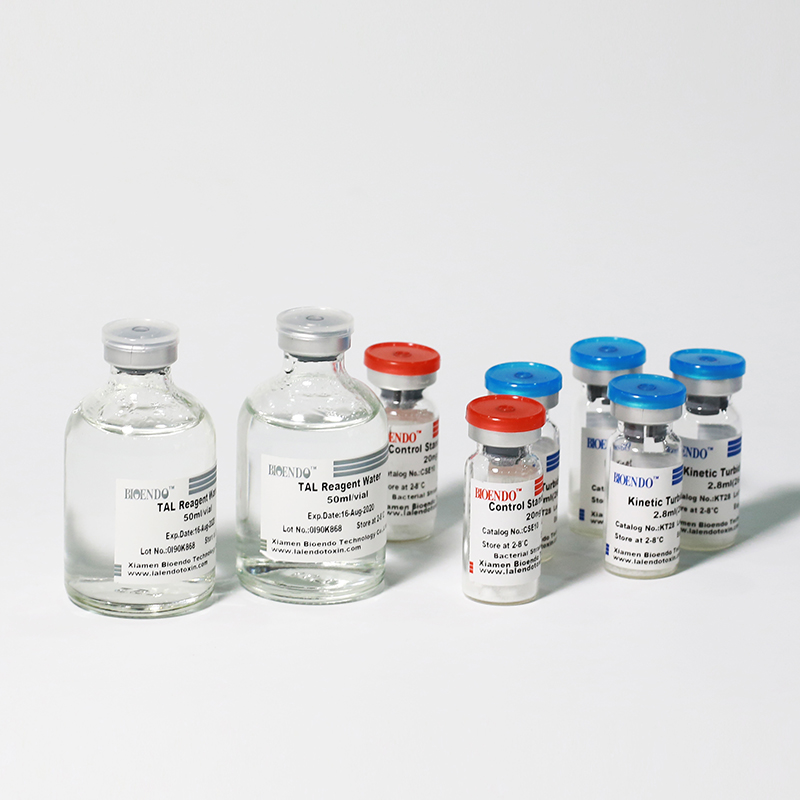
Kinetic Turbidimetric Amebocyte Lysate Vial
1. Product Introduction
Kinetic Turbidimetric Amebocyte Lysate Vial is developed based on the principle that the time needed to reach a certain absorbance increase (onset OD), i.e. onset time, is negatively correlated with the endotoxin concentration. Sensitivity could reach 0.005EU/ml, and the detection could reach four orders of magnitude. It is specially suitable for pharmaceuticals industry to monitor endotoxin concentration.
2. Product Parameter:
Assay range:0.005-50EU/ml; 0.01 – 10EU/ml
3. Product Application
End-product endotoxin (pyrogen) qualification, Water for injection endotoxin assay, raw material endotoxin testing or endotoxin level monitoring during manufacturing process for pharmaceutical companies or medical devices manufacturers.
Note:
Lyophilized Amebocyte Lysate reagent manufactured by Bioendo is made from amebocyte lysate from the horseshoe crab (Tachypleus tridentatus).
|
Catalog No. |
ml/vial |
Tests/Vial |
Vials/Pack |
Sensitivity EU/ml |
|
KT17 |
1.7 |
16 |
10 |
0.01-10EU/ml |
|
KT17S |
1.7 |
16 |
10 |
0.005-5EU/ml, 0.01-10EU/ml |
|
KT52 |
5.2 |
50 |
10 |
0.01-10EU/ml |
|
KT52S |
5.2 |
50 |
10 |
0.005-5EU/ml, 0.01-10EU/ml |
The Lyophilized Amebocyte Lysate reagent sensitivity and the Control Standard Endotoxin potency are assayedagainst USP Reference Standard Endotoxin. The Lyophilized Amebocyte Lysate reagent kits come withproduct instruction, Certificate of Analysis.
Product detail pictures:


Related Product Guide:
Our corporation puts emphasis about the administration, the introduction of talented staff, plus the construction of team building, attempting hard to improve the quality and liability consciousness of team members. Our organization successfully attained IS9001 Certification and European CE Certification of Factory wholesale Kinetic Turbidimetric LAL Assay - Kinetic Turbidimetric Amebocyte Lysate Vial – Bioendo , The product will supply to all over the world, such as: Sacramento, India, Poland, Really should any of these items be of interest to you, please let us know. We will be pleased to give you a quotation upon receipt of one's detailed specifications. We've our personal specialist R&D enginners to meet any of the requriements, We look forward to receiving your enquires soon and hope to have the chance to work together with you inside the future. Welcome to take a look at our organization.
The after-sale warranty service is timely and thoughtful, encounter problems can be resolved very quickly, we feel reliable and secure.








