Free sample for Bakteriyel Endotoksin Testi Nasıl Yapılır - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo
Free sample for Bakteriyel Endotoksin Testi Nasıl Yapılır - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo Detail:
Single-Channel Mechanical Pipettes (Semi-sterile)
1. Product Information
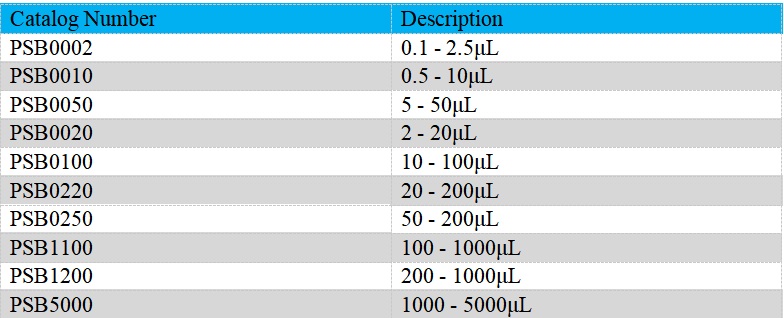
The Single-Channel Mechanical Pipettes (Semi-sterile) is adjustable, which could be used to dispense liquid precisely. Measuring volume of our Single-Channel Mechanical Pipettes (Semi-sterile) ranges from 0.1μL to 5mL. Products are produced based on ISO8655/DIN12650. It could be widely employed on endotoxin detection, etc..
2. Product Features
- Light weight, economic
- The pipettes cover volume range from 0.1μL to 5mL
- Semi-sterile
- Easy calibration and maintenance
Product detail pictures:

Related Product Guide:
"Sincerity, Innovation, Rigorousness, and Efficiency" will be the persistent conception of our company to the long-term to establish together with customers for mutual reciprocity and mutual gain for Free sample for Bakteriyel Endotoksin Testi Nasıl Yapılır - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo , The product will supply to all over the world, such as: Russia, Haiti, British, With its rich manufacturing experience, high-quality products, and perfect after-sale service, the company has gained good reputation and has become one of the famous enterprise specialized in manufacturing series.We sincerely hope to establish business relation with you and pursue mutual benefit.
Production management mechanism is completed, quality is guaranteed, high credibility and service let the cooperation is easy, perfect!