Free sample for Bakteriyel Endotoksin Testi Nasıl Yapılır - Single-Channel Mechanical Pipettor – Bioendo
Free sample for Bakteriyel Endotoksin Testi Nasıl Yapılır - Single-Channel Mechanical Pipettor – Bioendo Detail:
Single-Channel Mechanical Pipettor
1. Product Information
Single channel mechanical pipette is ideal tool to support endotoxin detection with Lyophilized Amebocyte Lysate which covers gel-clot technique, kinetic turbidimetric technique, kinetic chromogenic technique, and end-point chromogenic technique. All pipettors are produced by following ISO8655 – 2:2002. The quality control involves gravimetric testing of each pipette with distilled water at 22℃.
2. Product Features:
- Light weight, economic, low force design
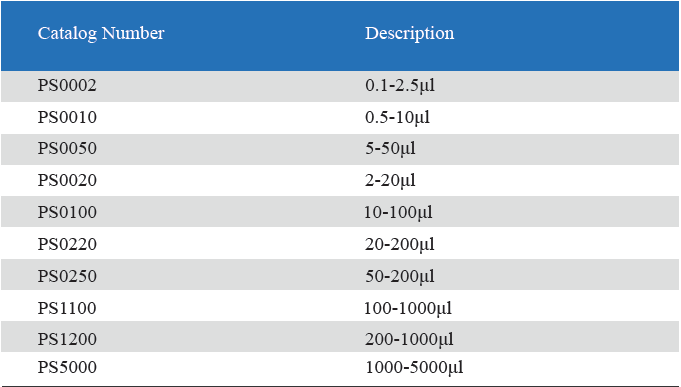
- Measuring volume range from 0.1μL to 5mL
- Easy to calibrate and maintain with tool supplied
- Design helps to avoid repetitive strain injuries
- Calibrated in accordance with ISO8655. Each pipettor supplied with individual test certificate
- The low part is available for autoclaving
Product detail pictures:

Related Product Guide:
Our goods are broadly recognized and reliable by users and can meet consistently switching financial and social demands of Free sample for Bakteriyel Endotoksin Testi Nasıl Yapılır - Single-Channel Mechanical Pipettor – Bioendo , The product will supply to all over the world, such as: Israel, Bulgaria, Boston, We firmly think that we have the full capability to present you contented merchandise. Wish to collect concerns within you and build a new long-term synergy romantic relationship. We all significantly promise:Csame excellent, better selling price; exact selling price, better quality.
The company keeps to the operation concept "scientific management, high quality and efficiency primacy, customer supreme", we have always maintained business cooperation. Work with you,we feel easy!








