Good quality Chromogenic Endotoxin Quant Kit - Endpoint Chromogenic Kit EC80545 – Bioendo
Good quality Chromogenic Endotoxin Quant Kit - Endpoint Chromogenic Kit EC80545 – Bioendo Detail:
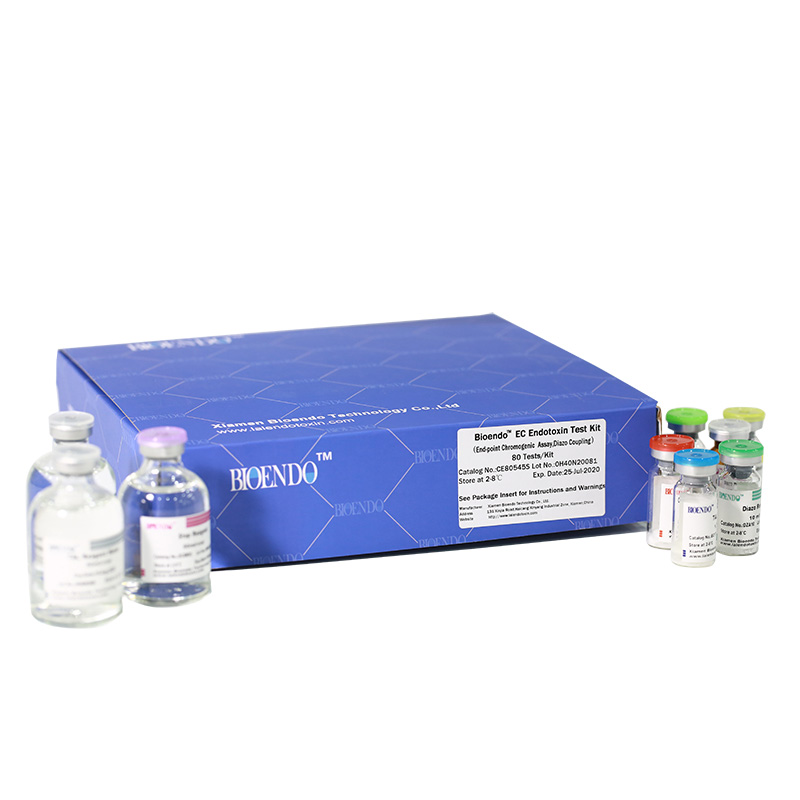
Bioendo EC Endotoxin Test Kit (End-point Chromogenic Assay, Diazo Coupling)
1. Product Information
Bioendo EC Endotoxin Test Kit (End-point Chromogenic Assay, Diazo Coupling) provides a fast measurement for endotoxin quantification. The endotoxin in the sample activates a cascade of enzymes in Amebocyte Lysate, the activated enzyme splits the synthetic substrate, releasing a yellow-colored item. Then the yellow item can further react with diazo reagents to form purple items with maximum absorbance at 545nm. A regular spectrophotometer or microplate reader is required for the assay. The purple items are proportional to endotoxin concentration. Then endotoxin testing result is quantitative analysis.
2. Product Parameter
Sensitivity Range: 0.01-0.1EU/ml (assay time about 46 minutes)
0.1-1.0EU/ml (assay time about 16 minutes)
3. Product Features and Application
Bioendo EC Endotoxin Test Kit (End-point Chromogenic Assay, Diazo Coupling) is intended for use in the In Vitro detection and quantitation of gram-negative bacterial endotoxins. The colorless artificial peptide substrate solution is added into Lyophilized Amebocyte Lysate, and then test specimen mixture. If specimen contains endotoxin, color of specimen mixture changes. The absorbance is related to the endotoxin concentration. Therefore the endotoxn levels in specimen mixture can be claculated against a standard curve. Standard spectrophotometer with 540 – 545nm filter is enough to quantify endotoxin with our EC Endotoxin Test Kit (End-point Chromogenic Assay, Diazo Coupling).
Note:
Lyophilized Amebocyte Lysate (LAL) reagent manufactured by Bioendo is made from amebocyte lysate derived blood of horseshoe crab.
|
Catalog No. |
Description |
Kit Contents |
Sensitivity EU/ml |
|
EC80545 |
Bioendo™ EC Endotoxin Test Kit (End-point Chromogenic Assay, Diazo Coupling), 80 Tests/Kit |
5 Lyophilized Amebocyte Lysate, 1.7ml/vial; 4 Water for BET, 50ml/vial; 5 CSE; 5 Chromogenic Substrate, 1.7ml/vial; 5 Diazo Reagent 1, 10ml/vial; 5 Diazo Reagent 2, 10ml /vial; 5 Diazo Reagent 3, 10ml/vial; |
0.1 – 1 EU/ml |
|
EC80545S |
0.01 – 0.1 EU/ml; 0.1 – 1 EU/ml |
The sensitivity of Lyophilized Amebocyte Lysate and potency of Control Standard Endotoxin are assayed against USP Reference Standard Endotoxin. The Lyophilized Amebocyte Lysate reagent kits come with product instruction, Certificate of Analysis.
Does the End point endotoxin test kit need sophisticated microplate reader?
Bioendo EC80545 and EC80545S, can read by regular spectrophotometer.
Product detail pictures:




Related Product Guide:
We believe that long time period partnership is a result of top of the range, value added services, rich expertise and personal contact for Good quality Chromogenic Endotoxin Quant Kit - Endpoint Chromogenic Kit EC80545 – Bioendo , The product will supply to all over the world, such as: Colombia, Brisbane, Pakistan, Customer's satisfaction is always our quest, creating value for customers is always our duty, a long term mutual-beneficial business relationship is what we are doing for. We are an absolutely reliable partner for yourself in China. Of course, other services, like consulting, can be offered too.
We have been looking for a professional and responsible supplier, and now we find it.