High definition Pipette tip - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo
High definition Pipette tip - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo Detail:
Single-Channel Mechanical Pipettes (Semi-sterile)
1. Product Information
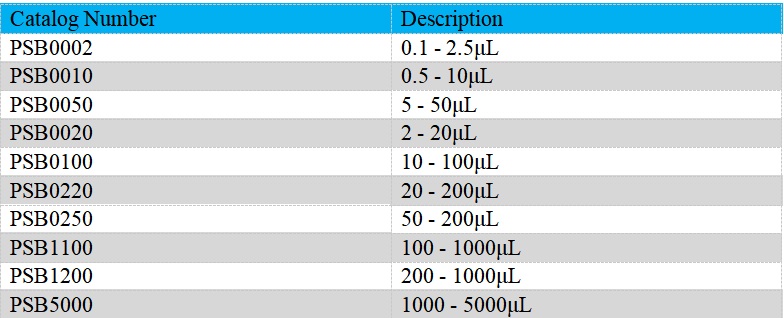
The Single-Channel Mechanical Pipettes (Semi-sterile) is adjustable, which could be used to dispense liquid precisely. Measuring volume of our Single-Channel Mechanical Pipettes (Semi-sterile) ranges from 0.1μL to 5mL. Products are produced based on ISO8655/DIN12650. It could be widely employed on endotoxin detection, etc..
2. Product Features
- Light weight, economic
- The pipettes cover volume range from 0.1μL to 5mL
- Semi-sterile
- Easy calibration and maintenance
Product detail pictures:

Related Product Guide:
"Quality initial, Honesty as base, Sincere support and mutual profit" is our idea, so as to build repeatedly and pursue the excellence for High definition Pipette tip - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo , The product will supply to all over the world, such as: Nairobi, Sao Paulo, Romania, With a team of experienced and knowledgeable personnel, our market covers South America, the USA, the Mid East, and North Africa. Many customers have become our friends after good cooperation with us. If you have the requirement for any of our products, please contact us now. We are looking forward to hearing from you soon.
We always believe that the details decides the company's product quality, in this respect, the company conform our requirements and the goods are meet our expectations.







