High Performance Endotoxin Detection Method - Depyrogenated Sample Bottles ( Endotoxin Free ) – Bioendo
High Performance Endotoxin Detection Method - Depyrogenated Sample Bottles ( Endotoxin Free ) – Bioendo Detail:
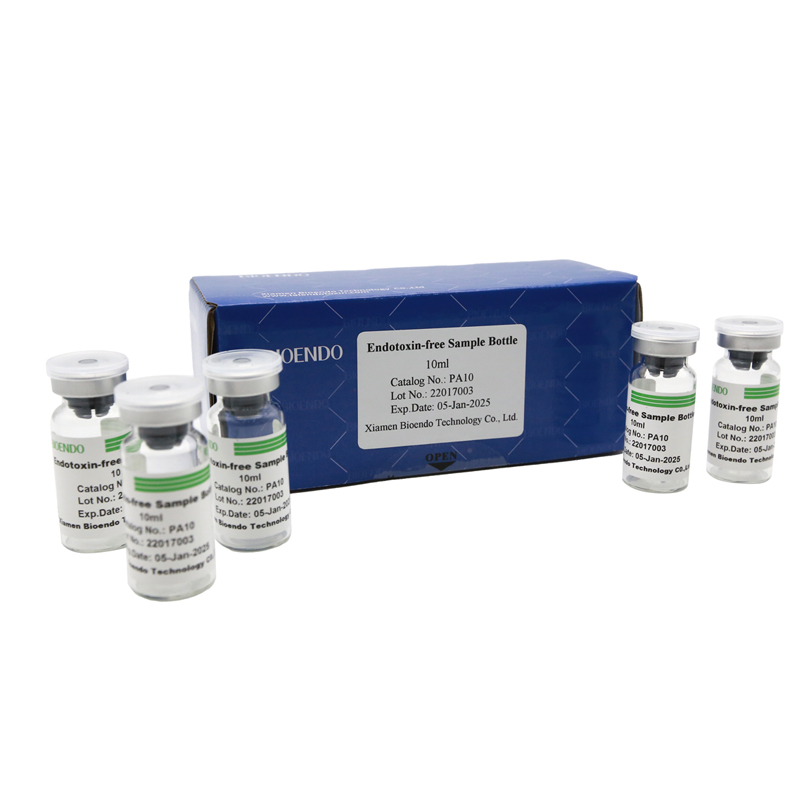
Depyrogenated Sample Container
1. Product information
We offer various of low endotoxin, pyrogen free accessories products,includes Water for Bacterial Endotoxins Test, pyrogen-free test tubes, pyrogen-free pipettor tips, microplates and sample bottles for your conveniences. High quality depyrogenated low endotoxin pyrogen free products insure the success of your experiments.
Depyrogenated (Endotoxin Free) Sample container(endotoxin free bottle, pyrogen free bottle, pyrogen free sample bottle)are glass bottles contain less than 0.005 EU/ml endotoxin. These bottles could be used to store various samples for lps endotoxin test, such as protein solution, vaccines, DNA solutions, dialysate, water for injections, etc., for endotoxin testing. Come with endotoxin free seals.
2. Product parameter
Endotoxin level < 0.005 EU/ml
3. Product features and application
For preparing and storage of the test samples.
| Catalog Number | Descriptions | Package |
| PA2 | Endotoxin free sample glass ampoule, 2ml | 10Pcs/Pack |
| PA10 | Endotoxin free sample glass vial, 10ml | 10Pcs/Pack or 110Pcs/Pack |
| PA50 | Endotoxin free sample glass vial, 50ml | 10Pcs/Pack |
| PA125 | Pyrogen free sample bottle, 125ml | 1Pcs/Pack |
| PA500 | Pyrogen free sample bottle, 500ml | 1Pcs/Pack |
such as endotoxin free tubes; endotoxin free tips; endotoxin free microplates; endotoxin free sample bottles;
According to China Pharmacopoeia, the utensils needed in the procedure of endotoxin test assay, such as sample vessel, dilution and reaction tubes, pipette tips, have to choose endotoxin free consumables. the utensils needed for the experiment need to be processed to remove possible exogenous endotoxins. If the endotoxin is not removed, it will interfere with the experiment.
Product detail pictures:





Related Product Guide:
We know that we only thrive if we will guarantee our combined cost competiveness and high-quality advantageous at the same time for High Performance Endotoxin Detection Method - Depyrogenated Sample Bottles ( Endotoxin Free ) – Bioendo , The product will supply to all over the world, such as: Porto, UK, Islamabad, The development of our company not only needs the guarantee of quality, reasonable price and perfect service, but also relies on our customer's trust and support! In the future, we are going to continue with the most qualified and high quality service to offer the most competitive price, Together with our customers and achieve win-win! Welcome to inquiry and consult!
In general, we are satisfied with all aspects, cheap, high-quality, fast delivery and good procuct style, we will have follow-up cooperation!








