High Quality LAL test gel clot - Gel Clot Lyophilized Amebocyte Lysate Single Test in Ampoule – Bioendo
High Quality LAL test gel clot - Gel Clot Lyophilized Amebocyte Lysate Single Test in Ampoule – Bioendo Detail:
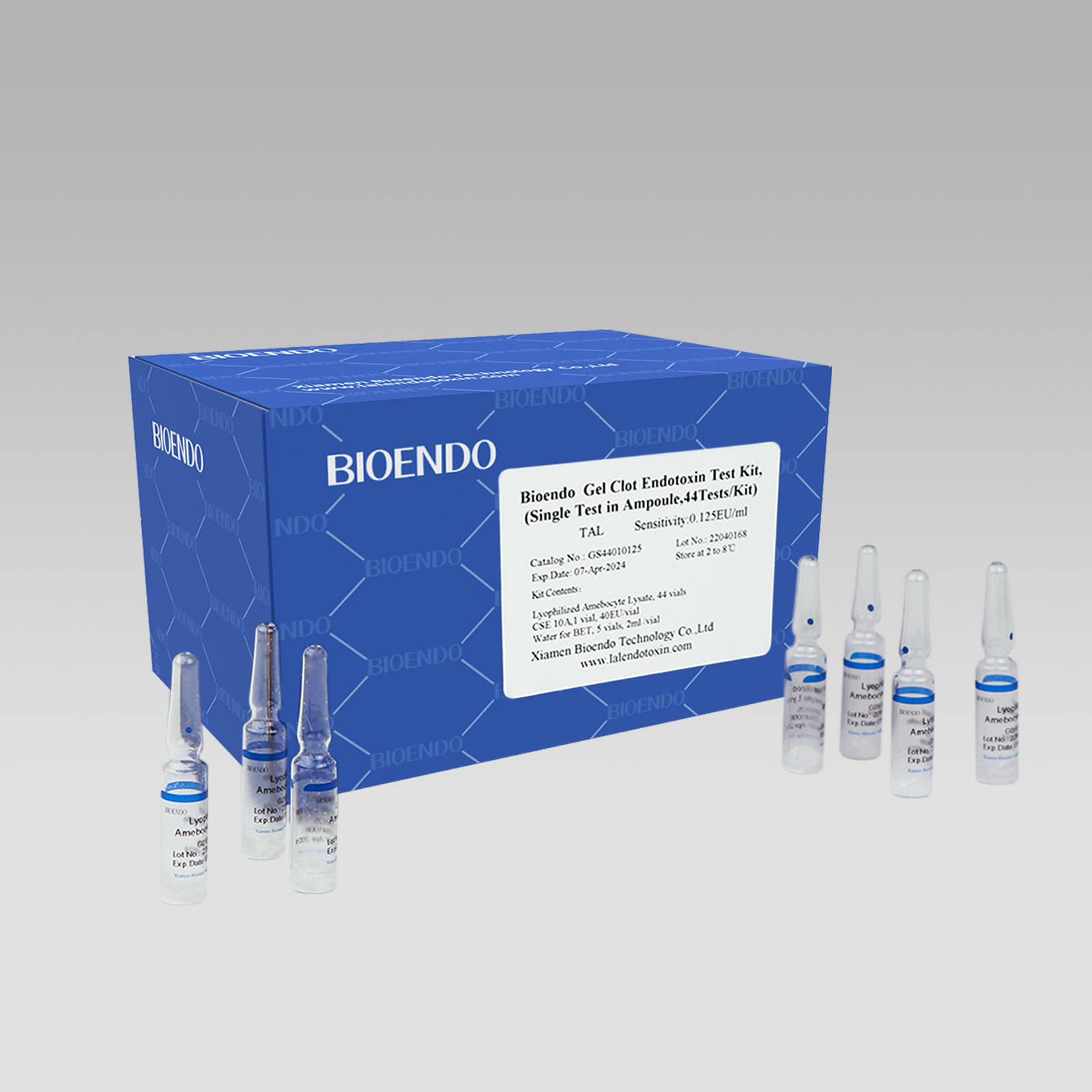
Gel Clot Lyophilized Amebocyte Lysate Single Test in Ampoule
1. Product Information
Gel Clot Lyophilized Amebocyte Lysate Single Test in ampoule Contain endotoxin-specific Amebocyte Lysate which includes beta-glucan inhibitor in the formulation and will not react to beta-glucan. For our Single Test in glass ampoule, you could add sample to the glass ampoules directly. This means you don’t need to reconstitute Amebocyte Lysate at first, and you will decide how many tests use each time to avoid waste. Endotoxin free tubes for the dilution of Lyophilized CSE is necessary. Operation of endotoxin detection use Bioendo Gel Clot Lyophilized Amebocyte Lysate Single Test in ampoule conforms to the USP, the EP.
2. Product Parameter
Gel clot assay single test glass ampoule.
Sensitivities: 0.03EU/ml, 0.06EU/ml, 0.125EU/ml, 0.25 EU/ml
3.Product Feature and Application
Single step endotoxin detection,
without expensive endotoxin assay instrument.
Suitable for end-product endotoxin testing before product released.
Product sensitivity standardized according to US Pharmacopoeia criterion and China Pharmacopoeia criterion.
Note:
Lyophilized Amebocyte Lysate (LAL) reagent manufactured by Bioendo is made from lysate of amebocytes (white blood cells) from the horseshoe crab.
|
Catalog No. |
Sensitivity EU/ml |
Description |
Kit Contents |
|
GS44010030 |
0.03 |
Bioendo Gel Clot Endotoxin Test Kit, (Single Test in Ampoule, 44Tests/Kit), |
44 Gel Clot Lyophilized Amebocyte Lysate; 1 CSE 10A; 5 Water for BET , 2ml/vial |
|
GS44010060 |
0.06 |
||
|
GS44010125 |
0.125 |
||
|
GS44010250 |
0.25 |
||
|
GS44010500 |
0.5 |
Product Condition
The sensitivity of Lyophilized Amebocyte Lysate and potency of Control Standard Endotoxin are assayed against USP Reference Standard Endotoxin. The Lyophilized Amebocyte Lysate reagent kits come with product instruction, Certificate of Analysis, MSDS.
Why the most selected gel clot assay kit GS44:
1. The most economical and commonly used test reagent for endotoxin detection.
2. Single test in a vial for one stepping reduce the risk of contamination.
3. Gel clot assay single test glass vial no need sophisticated microplate reader.
4. Saving endotoxin free tube when using GS44 series to test endotoxins in the procedure of endotoxin test assay’s operation.
Product detail pictures:
Related Product Guide:
We often stay with the principle "Quality Very first, Prestige Supreme". We have been fully committed to supplying our consumers with competitively priced high-quality goods, prompt delivery and skilled provider for High Quality LAL test gel clot - Gel Clot Lyophilized Amebocyte Lysate Single Test in Ampoule – Bioendo , The product will supply to all over the world, such as: Cancun, Canada, Vietnam, Upon today, we've customers from all over the world, including USA, Russia, Spain, Italy, Singapore, Malaysia, Thailand, Poland, Iran and Iraq. The mission of our company is to provide the highest quality products with best price. We've been looking forward to doing business with you.
Timely delivery, strict implementation of the contract provisions of the goods, encountered special circumstances, but also actively cooperate, a trustworthy company!








