Hot New Products Kinetic chromogenic TAL test kit - Endpoint Chromogenic Kit EC64405 – Bioendo
Hot New Products Kinetic chromogenic TAL test kit - Endpoint Chromogenic Kit EC64405 – Bioendo Detail:
End-point Chromogenic Endotoxin Test Kit (without Diazo Coupling)
1. Product Information
End-point Chromogenic Endotoxin Test Kit (without Diazo Coupling) is conducted by adding a colorless artificial peptide substrate solution into mixture of Lyophilized Amebocyte Lysate and test sample after a certain incubation period. If test sample contain endotoxin, a yellow color will develop in the 96 well microplate. Its absorbance (λmax = 405nm) is related to the endotoxin concentration. Endotoxin concentration of test sample could be calculated based on a standard curve.
2. Product parameter
Sensitivity Range: 0.01-0.1 EU/ml, matching assay time is about 46 minutes
0.1-1 EU/ml, matching assay time is about 16 minutes.
3. Product Features and Application
End-point Chromogenic Endotoxin Test Kit (without Diazo Coupling) is intended for use in the In Vitro detection and quantitation of gram-negative bacterial endotoxins. The colorless artificial peptide substrate solution is added into the mixture of Lyophilized Amebocyte Lysate and test sample after a certain incubation period. Yellow color develops if test sample contains endotoxin. Then use regular spectrophotometer or plate reader to read the absorbance at 405nm. End-point Chromogenic Endotoxin Test Kit (without Diazo Coupling) is suitable for all kinds of biomedical samples such as protein, vaccine, plasmid, DNA, RNA endotoxin assay.
Note:
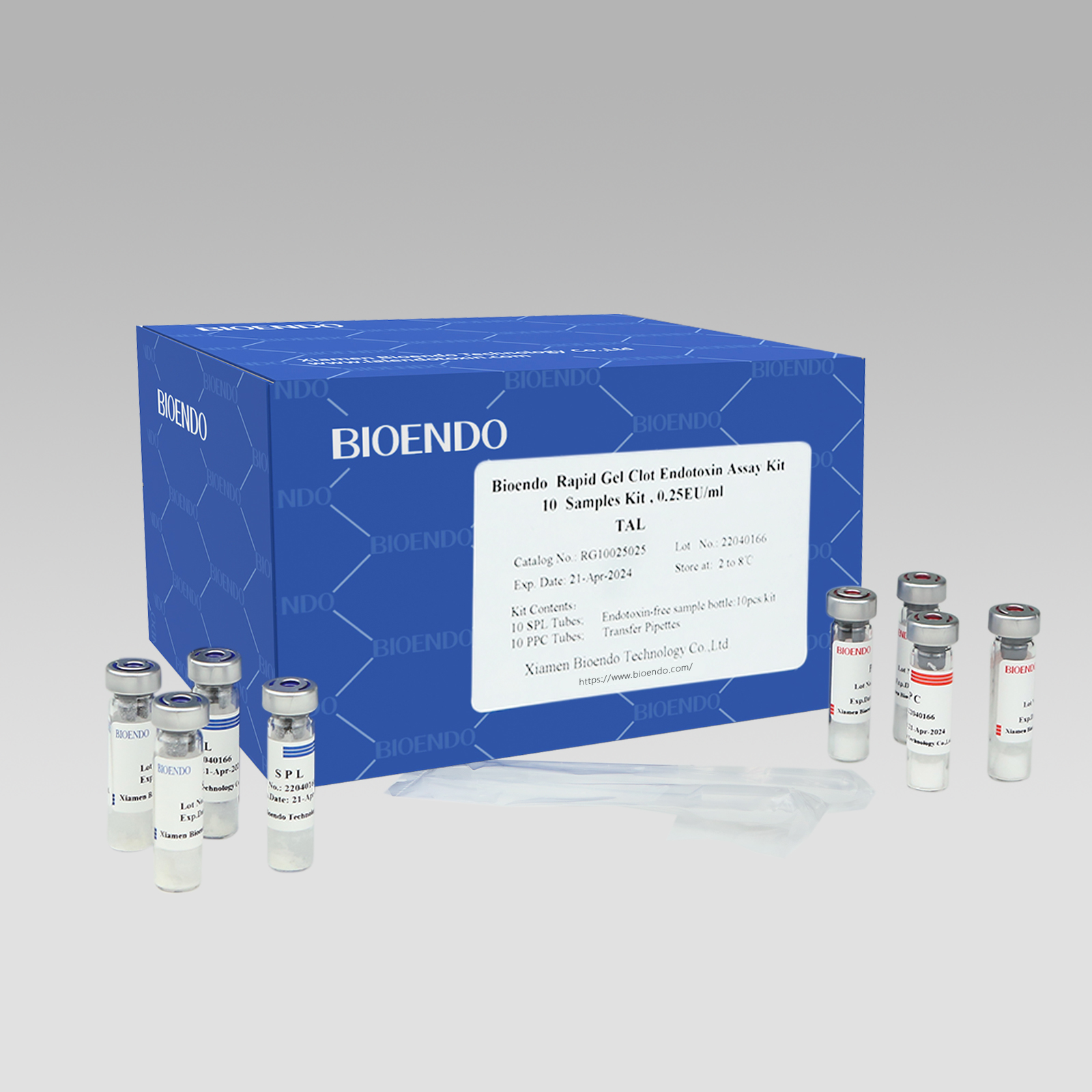
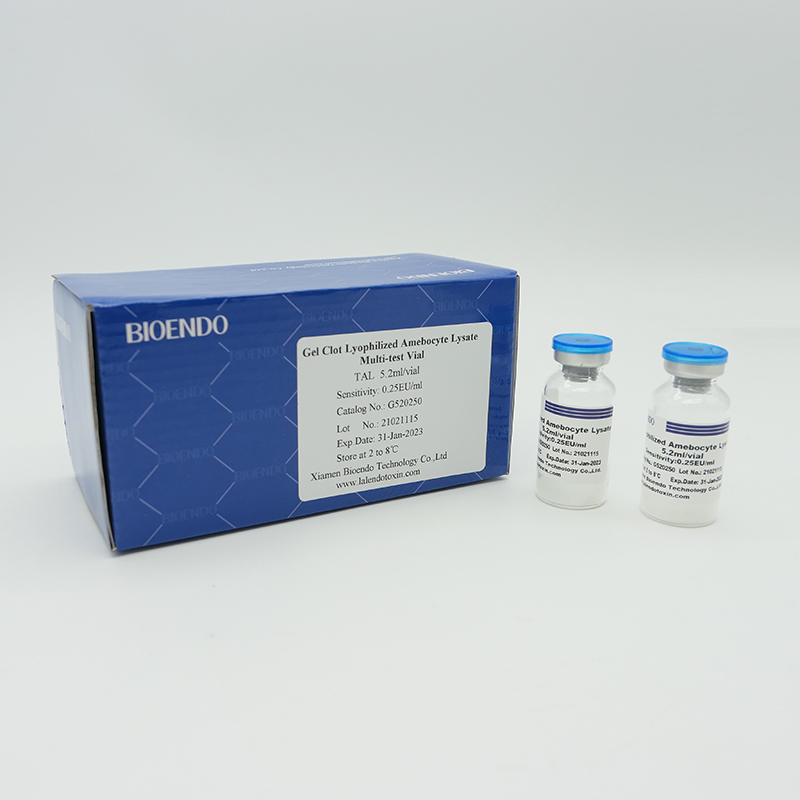
Lyophilized Amebocyte Lysate (LAL) reagent manufactured by Bioendo is made from amebocyte lysate derived blood of horseshoe crab.
|
Catalog No. |
Description |
Kit Contents |
Sensitivity (EU/ml) |
|
EC64405 |
Bioendo™ EC Endotoxin Test Kit (End-point Chromogenic Assay), 64 Tests/Kit
|
2 Lyophilized Amebocyte Lysate, 1.7ml/vial; 2 Water for BET, 50ml/vial; 2 CSE; 4 Chromogenic Substrate, 1.7ml/vial; |
0.1 – 1 EU/ml |
|
EC64405S |
0.01 – 0.1 EU/ml; 0.1 – 1 EU/m |
The sensitivity of Lyophilized Amebocyte Lysate and potency of Control Standard Endotoxin are assayed against USP Reference Standard Endotoxin. The Lyophilized Amebocyte Lysate reagent kits come with product instruction, Certificate of Analysis.
Does the End point endotoxin test kit need sophisticated microplate reader?
Bioendo EC64405 and EC64405S kit analysis the quantitative endotoxin results through regular microplate reader.
Endotoxin Free Tube
Pyrogen Free Tips
Pyrogen Free Microplates
Regular Microplate reader
Matched Control standard endotoxin (CSE10V)
BET water use for reconstitution.
Product detail pictures:




Related Product Guide:
Our enterprise since its inception, constantly regards product good quality as organization life, constantly improve production technology, strengthen merchandise high quality and continuously strengthen enterprise total good quality administration, in strict accordance with all the national standard ISO 9001:2000 for Hot New Products Kinetic chromogenic TAL test kit - Endpoint Chromogenic Kit EC64405 – Bioendo , The product will supply to all over the world, such as: Gabon, Singapore, Malaysia, Whether selecting a current product from our catalog or seeking engineering assistance for your application, you can talk to our customer service center about your sourcing requirements. We can provided good quality with competitive price for you.
This is a very professional and honest Chinese supplier, from now on we fell in love with the Chinese manufacturing.