Hot New Products LAL reagent sensitivity - Eight-channel Mechanical Pipette – Bioendo
Hot New Products LAL reagent sensitivity - Eight-channel Mechanical Pipette – Bioendo Detail:
Eight-Channel Mechanical Pipettor
1. Product information
All multi-channel mechanical pipettor have been quality tested according to ISO8655-2:2002 with calibration certificate. The quality control involves gravimetric testing of each pipette with distilled water at 22℃. The multichannel mechanical pipettor is idea for the detectionof bacterial endotoxin lal endotoxin testing by kinetic turbidimetric andkinetic chromogenic method.
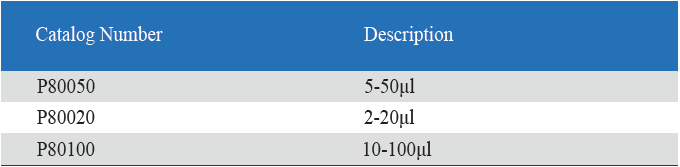
- Eight-Channel Mechanical Pipettor is available for standard 96-well plate
- Dispensing head rotates for optimum pipetting convenience
- Individual piston and tip cone assemblies allow easy repair and maintenance
- Compound material tip cone design allows visual seal verification
- Can be used with universal style pipette tips
-good for kinetic chromogenic, kinetic turbidimetric TAL orend-point chromogenic TAL endotoxin assay
Product detail pictures:
Related Product Guide:
Bear "Customer 1st, Good quality first" in mind, we work closely with our prospects and supply them with efficient and professional services for Hot New Products LAL reagent sensitivity - Eight-channel Mechanical Pipette – Bioendo , The product will supply to all over the world, such as: Afghanistan, Cyprus, United States, Our products are exported worldwide. Our customers are always satisfied with our reliable quality, customer-oriented services and competitive prices. Our mission is "to continue to earn your loyalty by dedicating our efforts to the constant improvement of our products and services in order to ensure the satisfaction of our end-users, customers, employees, suppliers and the worldwide communities in which we cooperate".
High production efficiency and good product quality, fast delivery and completed after-sale protection, a right choice, a best choice.








