Hot-selling LAL Turbidimetric test - Kinetic Turbidimetric Amebocyte Lysate Vial – Bioendo
Hot-selling LAL Turbidimetric test - Kinetic Turbidimetric Amebocyte Lysate Vial – Bioendo Detail:
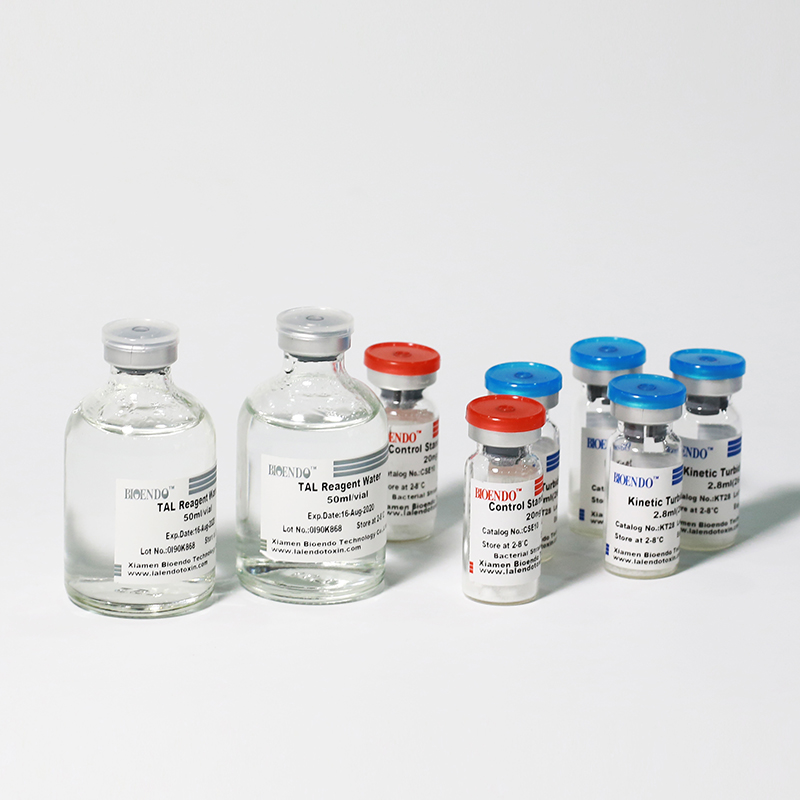
Kinetic Turbidimetric Amebocyte Lysate Vial
1. Product Introduction
Kinetic Turbidimetric Amebocyte Lysate Vial is developed based on the principle that the time needed to reach a certain absorbance increase (onset OD), i.e. onset time, is negatively correlated with the endotoxin concentration. Sensitivity could reach 0.005EU/ml, and the detection could reach four orders of magnitude. It is specially suitable for pharmaceuticals industry to monitor endotoxin concentration.
2. Product Parameter:
Assay range:0.005-50EU/ml; 0.01 – 10EU/ml
3. Product Application
End-product endotoxin (pyrogen) qualification, Water for injection endotoxin assay, raw material endotoxin testing or endotoxin level monitoring during manufacturing process for pharmaceutical companies or medical devices manufacturers.
Note:
Lyophilized Amebocyte Lysate reagent manufactured by Bioendo is made from amebocyte lysate from the horseshoe crab (Tachypleus tridentatus).
|
Catalog No. |
ml/vial |
Tests/Vial |
Vials/Pack |
Sensitivity EU/ml |
|
KT17 |
1.7 |
16 |
10 |
0.01-10EU/ml |
|
KT17S |
1.7 |
16 |
10 |
0.005-5EU/ml, 0.01-10EU/ml |
|
KT52 |
5.2 |
50 |
10 |
0.01-10EU/ml |
|
KT52S |
5.2 |
50 |
10 |
0.005-5EU/ml, 0.01-10EU/ml |
The Lyophilized Amebocyte Lysate reagent sensitivity and the Control Standard Endotoxin potency are assayedagainst USP Reference Standard Endotoxin. The Lyophilized Amebocyte Lysate reagent kits come withproduct instruction, Certificate of Analysis.
Product detail pictures:


Related Product Guide:
Our products are widely recognized and trusted by users and can meet continuously changing economic and social needs for Hot-selling LAL Turbidimetric test - Kinetic Turbidimetric Amebocyte Lysate Vial – Bioendo , The product will supply to all over the world, such as: Slovakia, Benin, Niger, By integrating manufacturing with foreign trade sectors, we can provide total customer solutions by guaranteeing the delivery of right products to the right place at the right time, which is supported by our abundant experiences, powerful production capability, consistent quality, diversified product portfolios and the control of the industry trend as well as our mature before and after sales services. We'd like to share our ideas with you and welcome your comments and questions.
Sales manager is very enthusiastic and professional, gave us a great concessions and product quality is very good,thank you very much!







