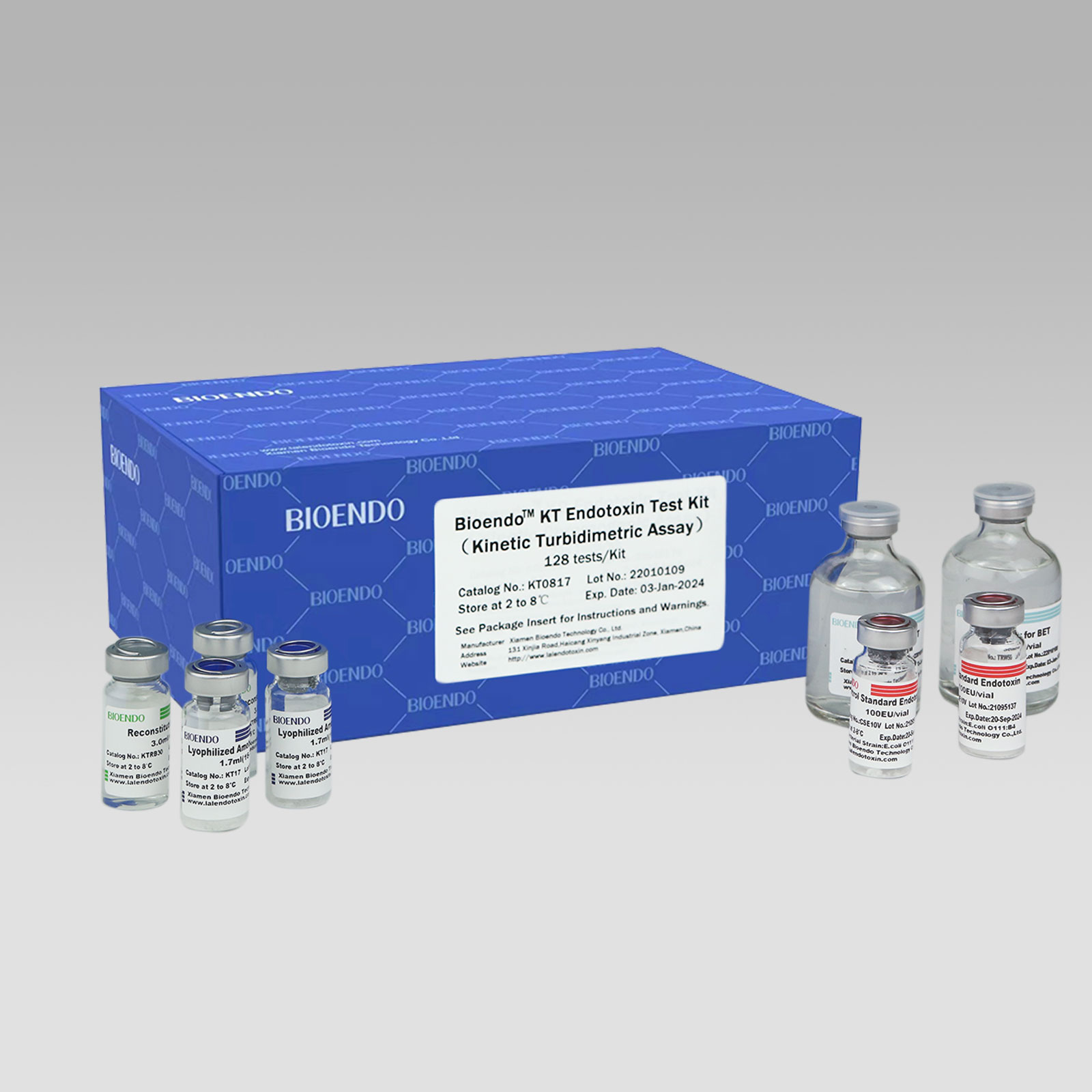
Low price for Bacterial Endotoxin Test Kinetic Turbidimetric Method – Kinetic Turbidimetric Amebocyte Lysate Vial – Bioendo
Low price for Bacterial Endotoxin Test Kinetic Turbidimetric Method – Kinetic Turbidimetric Amebocyte Lysate Vial – Bioendo Detail:
Kinetic Turbidimetric Amebocyte Lysate Vial
1. Product Introduction
Kinetic Turbidimetric Amebocyte Lysate Vial is developed based on the principle that the time needed to reach a certain absorbance increase (onset OD), i.e. onset time, is negatively correlated with the endotoxin concentration. Sensitivity could reach 0.005EU/ml, and the detection could reach four orders of magnitude. It is specially suitable for pharmaceuticals industry to monitor endotoxin concentration.
2. Product Parameter:
Assay range:0.005-50EU/ml; 0.01 – 10EU/ml
3. Product Application
End-product endotoxin (pyrogen) qualification, Water for injection endotoxin assay, raw material endotoxin testing or endotoxin level monitoring during manufacturing process for pharmaceutical companies or medical devices manufacturers.
Note:
Lyophilized Amebocyte Lysate reagent manufactured by Bioendo is made from amebocyte lysate from the horseshoe crab (Tachypleus tridentatus).
|
Catalog No. |
ml/vial |
Tests/Vial |
Vials/Pack |
Sensitivity EU/ml |
|
KT17 |
1.7 |
16 |
10 |
0.01-10EU/ml |
|
KT17S |
1.7 |
16 |
10 |
0.005-5EU/ml, 0.01-10EU/ml |
|
KT52 |
5.2 |
50 |
10 |
0.01-10EU/ml |
|
KT52S |
5.2 |
50 |
10 |
0.005-5EU/ml, 0.01-10EU/ml |
The Lyophilized Amebocyte Lysate reagent sensitivity and the Control Standard Endotoxin potency are assayedagainst USP Reference Standard Endotoxin. The Lyophilized Amebocyte Lysate reagent kits come withproduct instruction, Certificate of Analysis.
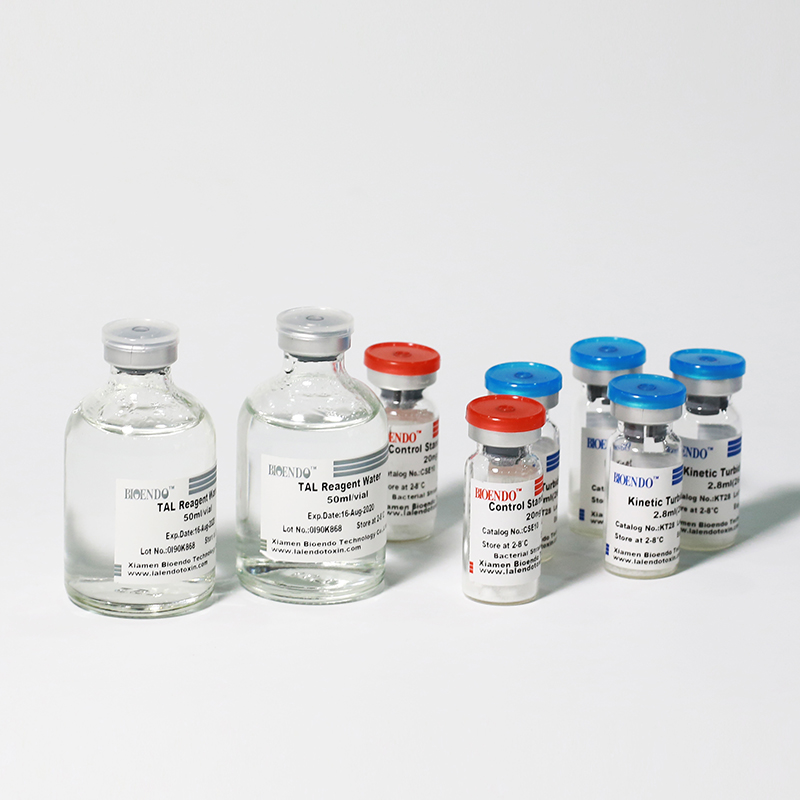
Product detail pictures:


Related Product Guide:
The corporation keeps to the operation concept "scientific management, superior quality and performance primacy, consumer supreme for Low price for Bacterial Endotoxin Test Kinetic Turbidimetric Method – Kinetic Turbidimetric Amebocyte Lysate Vial – Bioendo , The product will supply to all over the world, such as: Sheffield, Brisbane, Czech Republic, With the first-class products, excellent service, fast delivery and the best price, we have won highly praise foreign customers'. Our products have been exported to Africa, the Middle East, Southeast Asia and other regions.
The sales person is professional and responsible, warm and polite, we had a pleasant conversation and no language barriers on communication.