Manufacturer for LAL reagent manufacturer - Eight-channel Mechanical Pipette – Bioendo
Manufacturer for LAL reagent manufacturer - Eight-channel Mechanical Pipette – Bioendo Detail:
Eight-Channel Mechanical Pipettor
1. Product information
All multi-channel mechanical pipettor have been quality tested according to ISO8655-2:2002 with calibration certificate. The quality control involves gravimetric testing of each pipette with distilled water at 22℃. The multichannel mechanical pipettor is idea for the detectionof bacterial endotoxin lal endotoxin testing by kinetic turbidimetric andkinetic chromogenic method.
- Eight-Channel Mechanical Pipettor is available for standard 96-well plate
- Dispensing head rotates for optimum pipetting convenience
- Individual piston and tip cone assemblies allow easy repair and maintenance
- Compound material tip cone design allows visual seal verification
- Can be used with universal style pipette tips
-good for kinetic chromogenic, kinetic turbidimetric TAL orend-point chromogenic TAL endotoxin assay
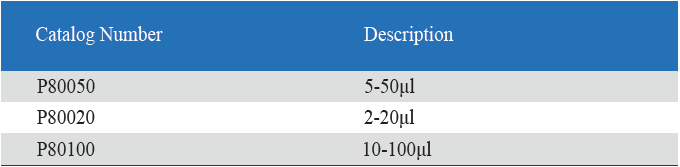
Product detail pictures:
Related Product Guide:
Innovation, excellent and reliability are the core values of our firm. These principles today more than ever form the basis of our success as an internationally active mid-size corporation for Manufacturer for LAL reagent manufacturer - Eight-channel Mechanical Pipette – Bioendo , The product will supply to all over the world, such as: Madrid, South Africa, Durban, Our items are widely recognized and trusted by users and can meet continuously developing economic and social needs. We welcome new and old customers from all walks of life to contact us for future business relationships and achieving mutual success!
Managers are visionary, they have the idea of "mutual benefits, continuous improvement and innovation", we have a pleasant conversation and Cooperation.







