Manufacturer of Dry Heat Sterilization Validation – Mini Dry Heat Incubator – Bioendo
Manufacturer of Dry Heat Sterilization Validation – Mini Dry Heat Incubator – Bioendo Detail:
Dry heat incubator single module
1. Product information
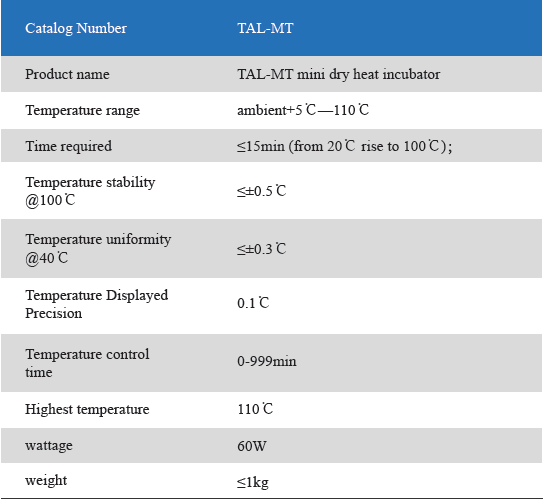
The Mini Dry Heat Incubator is a micro-processor controlled heating block with semi conductor heating technology.It adapts onboard use, smart, light and convenient for movement, suit for any kind of occasions. Especially good for the incubation of the gel clot LAL assay, LAL chromogenic endpoint assay incubation.
2. Product features
1. Unique designed. Smart and light, convenient movement, suit for various occasions.
2. LCD simultaneously display setting and actual time and temperature.Temperature calibration function.
3. Automatic fault detection function with buzzer alarm.
4. 24V DC input power, built-in over-temperature protection device.
5. Various of blocks for optional choice. Convenient for replacement. Easy cleaning and disinfection.
Product detail pictures:



Related Product Guide:
We thinks what buyers think, the urgency of urgency to act during the interests of a purchaser position of theory, allowing for much better high-quality, reduced processing costs, charges are more reasonable, won the new and outdated consumers the support and affirmation for Manufacturer of Dry Heat Sterilization Validation – Mini Dry Heat Incubator – Bioendo , The product will supply to all over the world, such as: Paraguay, Lithuania, Swedish, Our company upholds the spirit of "innovation, harmony, team work and sharing, trails, pragmatic progress". Give us a chance and we will prove our capability. With your kind help, we believe that we can create a bright future with you together.
This company can be well to meet our needs on product quantity and delivery time, so we always choose them when we have procurement requirements.








