Manufacturer of Dry Heat Sterilization Validation – Rapid Gel Clot 10 Samples Kit – Bioendo
Manufacturer of Dry Heat Sterilization Validation – Rapid Gel Clot 10 Samples Kit – Bioendo Detail:
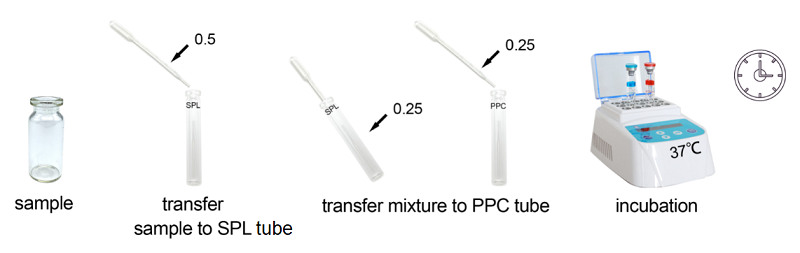
Bioendo Rapid Gel Clot Endotoxin Assay Kit is designed to rapidly quantify endotoxin in water or dialysate. Generally, RG kit’s result could be gained within 30 minutes. Under the guidance of detecting endotoxin in water or dialysate quickly, endotoxin detection with Bioendo Rapid Gel Clot Endotoxin Assay Kit does not need the multi steps’ dilution of Control Standard Endotoxin and test samples. Operation procedures are very convenient, additional experimental equipment are required. It is a convenient and rapid way to detect endotoxin in especial suitable for water or dialysate.
2. Product Parameter
Sensitivity Range: 0.03EU/ml, 0.06EU/ml, 0.125EU/ml, 0.25EU/ml, 0.5EU/ml
10 sample tests in the kit.
Assay time: less than 30 minutes
3. Product Application
Bioendo Rapid Gel Clot Endotoxin Assay Kit is designed to rapidly quantify endotoxin in water or dialysate as well as do quickly endotoxin detection in life science research.
Note:
Lyophilized Amebocyte Lysate (LAL) reagent manufactured by Bioendo is made from amebocyte lysate derived blood of horseshoe crab.
|
Catalog No. |
Description |
Kit Contents |
Sensitivity EU/ml |
Reaction Time minutes |
|
RG10025003 |
BioendoTM Rapid Gel Clot Endotoxin Assay Kit, 10 Samples Kit |
10 SPL Tubes; 10 PPC Tubes; 10 Endotoxin-free Sample Bottles; 10 Packs of (3pcs Transfer Pipettes) |
0.03 |
≤60 |
|
RG10025006 |
0.06 |
≤60 |
||
|
RG100250125 |
0.125 |
≤45 |
||
|
RG10025025 |
0.25 |
≤30 |
||
|
RG10025050 |
0.5 |
≤30 |
Product detail pictures:
Related Product Guide:
Our products are widely recognized and trusted by users and can meet continuously changing economic and social needs of Manufacturer of Dry Heat Sterilization Validation – Rapid Gel Clot 10 Samples Kit – Bioendo , The product will supply to all over the world, such as: British, Zimbabwe, Moscow, With the enterprising spirit of" high efficiency, convenience, practicality and innovation", and in line with such serving guidance of "good quality but better price, " and "global credit", we are striving to cooperate with the automobile parts companies all over the world to make a win-win partnership.
This manufacturers not only respected our choice and requirements, but also gave us a lot of good suggestions, ultimately, we successfully completed the procurement tasks.