Manufacturing Companies for Advantages Of LAL Test - Eight-channel Mechanical Pipette – Bioendo
Manufacturing Companies for Advantages Of LAL Test - Eight-channel Mechanical Pipette – Bioendo Detail:
Eight-Channel Mechanical Pipettor
1. Product information
All multi-channel mechanical pipettor have been quality tested according to ISO8655-2:2002 with calibration certificate. The quality control involves gravimetric testing of each pipette with distilled water at 22℃. The multichannel mechanical pipettor is idea for the detectionof bacterial endotoxin lal endotoxin testing by kinetic turbidimetric andkinetic chromogenic method.
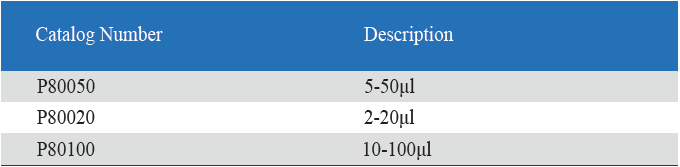
- Eight-Channel Mechanical Pipettor is available for standard 96-well plate
- Dispensing head rotates for optimum pipetting convenience
- Individual piston and tip cone assemblies allow easy repair and maintenance
- Compound material tip cone design allows visual seal verification
- Can be used with universal style pipette tips
-good for kinetic chromogenic, kinetic turbidimetric TAL orend-point chromogenic TAL endotoxin assay
Product detail pictures:
Related Product Guide:
The very rich projects management experiences and one to one service model make the high importance of business communication and our easy understanding of your expectations for Manufacturing Companies for Advantages Of LAL Test - Eight-channel Mechanical Pipette – Bioendo , The product will supply to all over the world, such as: Manila, Bahrain, Sweden, Our company will adhere to "Quality first, , perfection forever, people-oriented , technology innovation"business philosophy. Hard work to keep making progress, innovation in the industry, make every effort to first-class enterprise. We try our best to build the scientific management model, to learn abundant professional knowledge, to develop advanced production equipment and production process , to create the first-call quality products, reasonable price , high quality of service , quick delivery , to give you create new value .
The factory technical staff not only have high level of technology, their English level is also very good, this is a great help to technology communication.