New Arrival China Dry heat tunnel validation by ECV - Single-Channel Mechanical Pipettor – Bioendo
New Arrival China Dry heat tunnel validation by ECV - Single-Channel Mechanical Pipettor – Bioendo Detail:
Single-Channel Mechanical Pipettor
1. Product Information
Single channel mechanical pipette is ideal tool to support endotoxin detection with Lyophilized Amebocyte Lysate which covers gel-clot technique, kinetic turbidimetric technique, kinetic chromogenic technique, and end-point chromogenic technique. All pipettors are produced by following ISO8655 – 2:2002. The quality control involves gravimetric testing of each pipette with distilled water at 22℃.
2. Product Features:
- Light weight, economic, low force design
- Measuring volume range from 0.1μL to 5mL
- Easy to calibrate and maintain with tool supplied
- Design helps to avoid repetitive strain injuries
- Calibrated in accordance with ISO8655. Each pipettor supplied with individual test certificate
- The low part is available for autoclaving
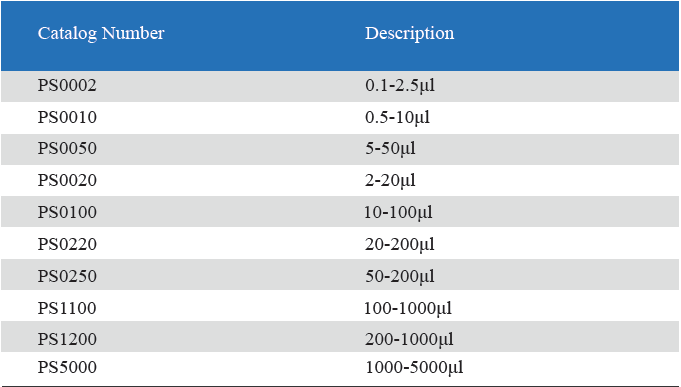
Product detail pictures:

Related Product Guide:
Our development depends on the advanced equipment ,excellent talents and continuously strengthened technology forces for New Arrival China Dry heat tunnel validation by ECV - Single-Channel Mechanical Pipettor – Bioendo , The product will supply to all over the world, such as: Cambodia, Qatar, Lesotho, We have a large share in global market. Our company has strong economic strength and offers excellent sale service. We have established faith, friendly, harmonious business relationship with customers in different countries. , such as Indonesia, Myanmar, Indi and other Southeast Asian countries and European, African and Latin American countries.
The company can think what our think, the urgency of urgency to act in the interests of our position, can be said this is a responsible company, we had a happy cooperation!