New Arrival China Kinetic Turbidimetric Method endotoxin - Bioendo KT Endotoxin Test Kit (Kinetic Turbidimetric Assay) – Bioendo
New Arrival China Kinetic Turbidimetric Method endotoxin - Bioendo KT Endotoxin Test Kit (Kinetic Turbidimetric Assay) – Bioendo Detail:
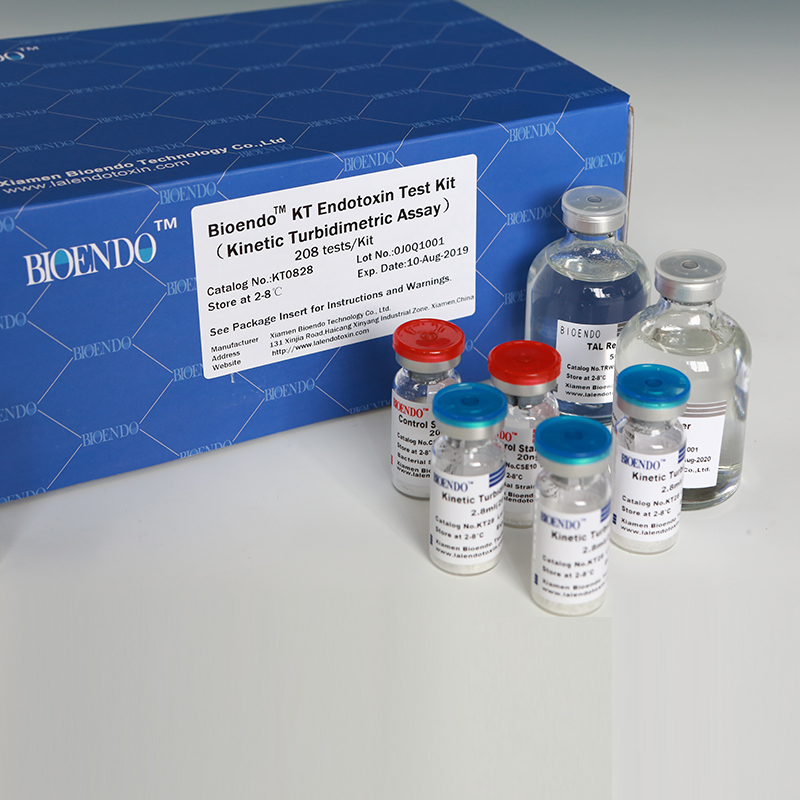
Bioendo KT Endotoxin Test Kit (Kinetic Turbidimetric Assay)
1. Product Introduction
Kinetic Turbidimetric Amebocyte Lysate Vial is developed based on the principle that the time needed to reach a certain absorbance increase (onset OD), i.e. onset time, is negatively correlated with the endotoxin concentration. Sensitivity could reach 0.005EU/ml, and the detection could reach four orders of magnitude. It is specially suitable for pharmaceuticals industry to monitor endotoxin concentration.
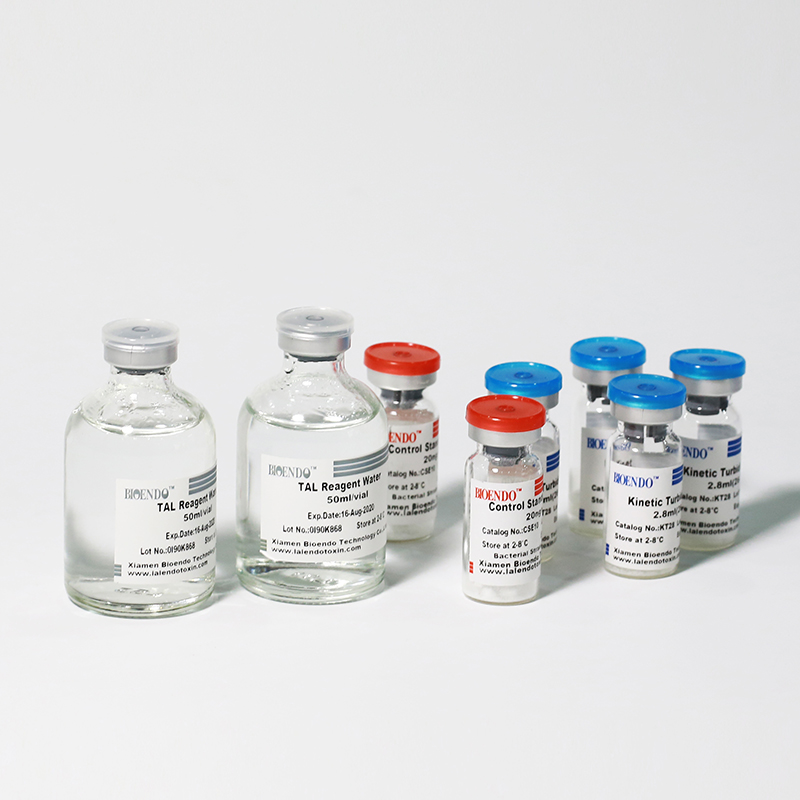
The kit contains Lyophilized Amebocyte Lysate, Control Standard Endotoxin, and Water for BET. KT Endotoxin Test Kit (Kinetic Turbidimetric Assay) require a kinetic microplate reader such as ELx808IULALXH or a kinetic tube reader. Kinetic software is also required for calculation of the endotoxin concentration.
2. Product Parameter
Assay Range: 0.005 – 5EU/ml; 0.01 – 10EU/ml
3. Product Application
End-product endotoxin (pyrogen) qualification, Waterfor injection endotoxin assay, raw material endotoxin testing or endotoxinlevel monitoring during manufacturing process for pharmaceutical companies ormedical devices manufacturers.
Note:
Lyophilized Amebocyte Lysate reagent manufactured by Bioendo is made from amebocyte lysate from the horseshoe crab (Tachypleus tridentatus).
|
Catalog No. |
Description |
Kit Contents |
Sensitivity EU/ml |
|
KT0817 |
Bioendo™ KT Endotoxin Test Kit (Kinetic Turbidimetric Assay), 128 tests/kit |
8 Lyophilized Amebocyte Lysate, 1.7ml (16 Tests/vial); 8 Reconstitution Buffer, 3.0ml/vial; 4 CSE; 2 Water for BET, 50ml/vial; |
0.01-10EU/ml |
|
KT0817S |
0.005-5EU/ml, 0.01-10EU/ml |
||
|
KT0852 |
Bioendo™ KT Endotoxin Test Kit (Kinetic Turbidimetric Assay), 400 Tests/Kit |
8 Lyophilized Amebocyte Lysate, 5.2ml (50 Tests/Vial); 8 Reconstitution Buffer, 6.0ml/vial; 4 CSE; 3 Water for BET, 50ml/vial; |
0.01-10EU/ml |
|
KT0852S |
0.005-5EU/ml, 0.01-10EU/ml |
||
|
KT5017 |
Bioendo™ KT Endotoxin Test Kit (Kinetic Turbidimetric Assay), 800 Tests/Kit |
50 Lyophilized Amebocyte Lysate, 1.7ml (16 Tests/vial); 50 Reconstitution Buffer, 3.0ml/vial; 10 CSE; |
0.01-10EU/ml |
|
KT5017S |
0.005-5EU/ml, 0.01-10EU/ml |
||
|
KT5052 |
Bioendo™ KT Endotoxin Test Kit (Kinetic Turbidimetric Assay), 2500 Tests/Kit |
50 Lyophilized Amebocyte Lysate,5.2ml (50 Tests/vial); 50 Reconstitution Buffer, 6.0ml/vial; 10 CSE; |
0.01-10EU/ml |
|
KT5052S |
0.005-5EU/ml, 0.01-10EU/ml |
Product Condition:
The potency of Lyophilized Amebocyte Lysate and Control Standard Endotoxin are assayed against USP Reference Standard Endotoxin. The Lyophilized Amebocyte Lysate reagent kits come with product instruction, Certificate of Analysis, MSDS.
Product detail pictures:



Related Product Guide:
We'll make each hard work to become excellent and excellent, and speed up our measures for standing from the rank of intercontinental top-grade and high-tech enterprises for New Arrival China Kinetic Turbidimetric Method endotoxin - Bioendo KT Endotoxin Test Kit (Kinetic Turbidimetric Assay) – Bioendo , The product will supply to all over the world, such as: New Zealand, Washington, Kenya, Our company has already had a lot of top factories and qualified technology teams in China, offering the best goods, techniques and services to worldwide customers. Honesty is our principle, skilled operation is our work, service is our goal, and customers' satisfaction is our future!
It is not easy to find such a professional and responsible provider in today's time. Hope that we can maintain long-term cooperation.








