New Fashion Design for Bacterial Endotoxin Test Method Validation Protocol - Control Standard Endotoxin (CSE) – Bioendo
New Fashion Design for Bacterial Endotoxin Test Method Validation Protocol - Control Standard Endotoxin (CSE) – Bioendo Detail:
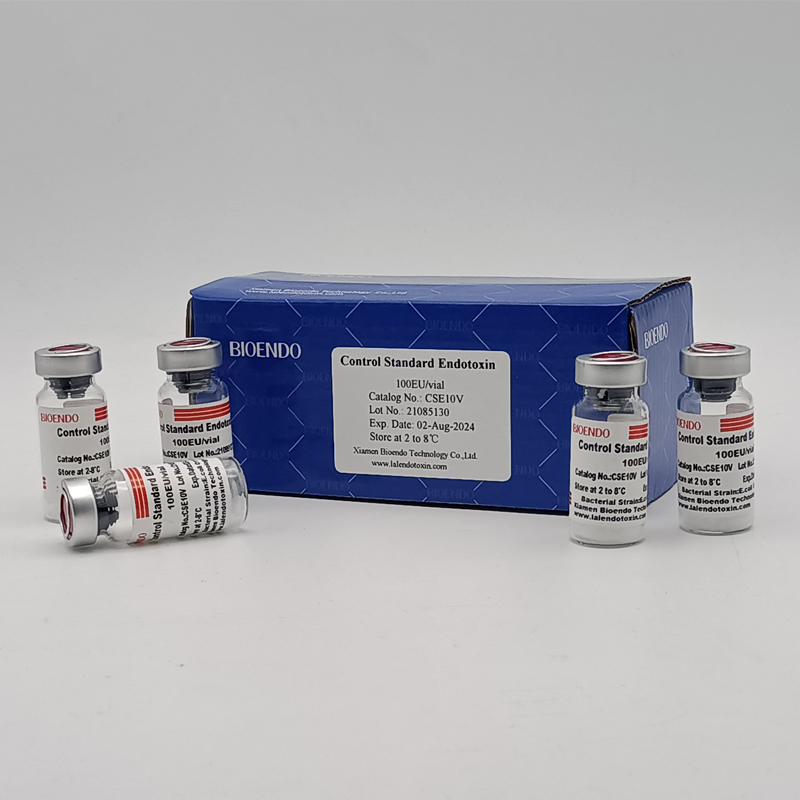
Control Standard Endotoxin (CSE)
1. Product Information
Control Standard Endotoxin (CSE) is extracted from E.coli O111:B4. CSE is an economic alternative to Reference Standard Endotoxin (RSE) in constructing standard curves, validating product and preparing controls in Lyophilized Amebocyte Lysate test. The labeled potency of CSE endotoxinE.coli standard is referenced against RSE. The Control Standard Endotoxin could be used with gel clot assay, kinetic turbidimetric assay or kinetic chromogenic assay as the endotoxin testing standards. The Certificate of Analysis will show the matched Lyophilized Amebocyte Lysate reagent lots.
2. Product Parameter
| Catalog Number | Potency (EU/vial) | Package |
| CSE10V | 100 to 999 EU | seal in glass vial, 10vials/pack |
| CSE100V | 1 to 199 EU | seal in glass vial, 10vials/pack |
| CSE10A | 1 to 99 EU | seal in glass ampoule, 10vials/pack |
3. Product Feature and Application
Bioendo CSE was labeled by the potency and matched to Lyophilized Amebocyte Lysate reagent lots. Users do not need to do the CSE/RSE ratio assay. Low potency control standard endotoxin is available to avoid lots of steps of dilution to provide convenience for end users.
Product Condition:
Control Standard Endotoxin (CSE), extracted from E.coli O111:B4, is an economic alternative to Reference Standard Endotoxin (RSE) in constructing standard curves, validating product and preparing controls in endotoxin test. The potency of CSE is referenced against USP Reference Standard Endotoxin, and labeled in the Certificate of Analysis.
Endotoxin test assay: Lysate reagent and CSE lot number have to be matched.
Pyrogen free tip box
Endotoxin free tubes
Product detail pictures:


Related Product Guide:
Quality First,and Customer Supreme is our guideline to provide the best service to our customers.Nowadays, we are trying our best to become one of the best exporters in our field to meet customers more need for New Fashion Design for Bacterial Endotoxin Test Method Validation Protocol - Control Standard Endotoxin (CSE) – Bioendo , The product will supply to all over the world, such as: Victoria, Finland, Kenya, We have been in operation for more than 10 years. We are dedicated to quality products and consumer support. We currently own 27 product utility and design patents. We invite you to visit our company for a personalized tour and advanced business guidance.
Adhering to the business principle of mutual benefits, we have a happy and successful transaction, we think we will be the best business partner.






