OEM Customized Bacterial Endotoxins Test - Control Standard Endotoxin (CSE) – Bioendo
OEM Customized Bacterial Endotoxins Test - Control Standard Endotoxin (CSE) – Bioendo Detail:
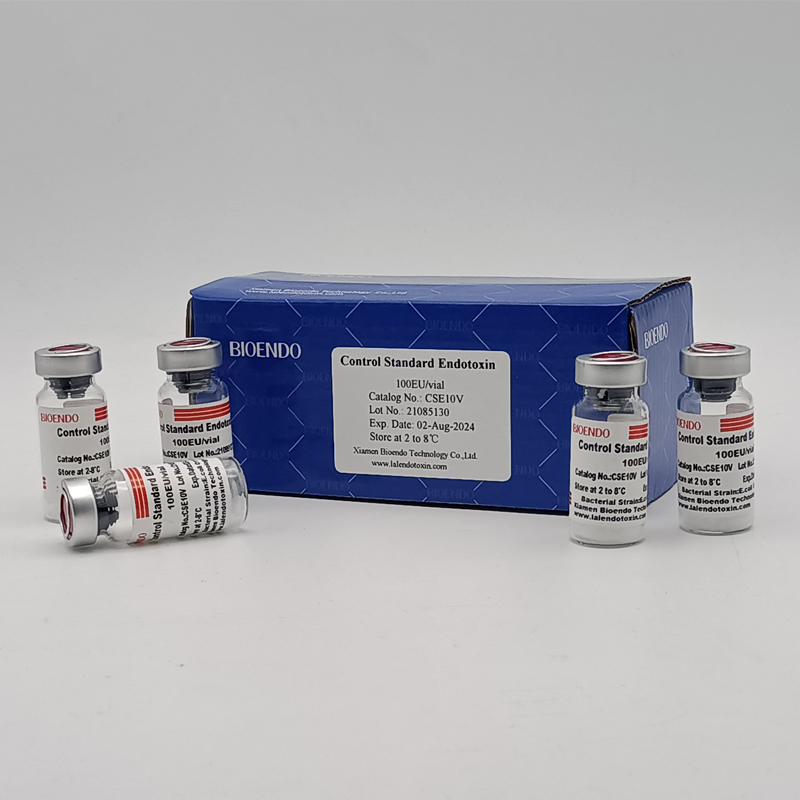
Control Standard Endotoxin (CSE)
1. Product Information
Control Standard Endotoxin (CSE) is extracted from E.coli O111:B4. CSE is an economic alternative to Reference Standard Endotoxin (RSE) in constructing standard curves, validating product and preparing controls in Lyophilized Amebocyte Lysate test. The labeled potency of CSE endotoxinE.coli standard is referenced against RSE. The Control Standard Endotoxin could be used with gel clot assay, kinetic turbidimetric assay or kinetic chromogenic assay as the endotoxin testing standards. The Certificate of Analysis will show the matched Lyophilized Amebocyte Lysate reagent lots.
2. Product Parameter
| Catalog Number | Potency (EU/vial) | Package |
| CSE10V | 100 to 999 EU | seal in glass vial, 10vials/pack |
| CSE100V | 1 to 199 EU | seal in glass vial, 10vials/pack |
| CSE10A | 1 to 99 EU | seal in glass ampoule, 10vials/pack |
3. Product Feature and Application
Bioendo CSE was labeled by the potency and matched to Lyophilized Amebocyte Lysate reagent lots. Users do not need to do the CSE/RSE ratio assay. Low potency control standard endotoxin is available to avoid lots of steps of dilution to provide convenience for end users.
Product Condition:
Control Standard Endotoxin (CSE), extracted from E.coli O111:B4, is an economic alternative to Reference Standard Endotoxin (RSE) in constructing standard curves, validating product and preparing controls in endotoxin test. The potency of CSE is referenced against USP Reference Standard Endotoxin, and labeled in the Certificate of Analysis.
Endotoxin test assay: Lysate reagent and CSE lot number have to be matched.
Pyrogen free tip box
Endotoxin free tubes
Product detail pictures:


Related Product Guide:
"Based on domestic market and expand abroad business" is our progress strategy for OEM Customized Bacterial Endotoxins Test - Control Standard Endotoxin (CSE) – Bioendo , The product will supply to all over the world, such as: Frankfurt, Swiss, Belize, Our business activities and processes are engineered to make sure our customers have access to widest range of products with the shortest supply time lines. This achievement is made possible by our highly skilled and experienced team. We look for people who want to grow with us around the globe and stand out from the crowd. We have people who embrace tomorrow, have vision, love stretching their minds and going far beyond what they thought was achievable.
The customer service reprersentative explained very detailed, service attitude is very good, reply is very timely and comprehensive, a happy communication! We hope to have a opportunity to cooperate.







