OEM Factory for BET Test Principle - Eight-channel Mechanical Pipette – Bioendo
OEM Factory for BET Test Principle - Eight-channel Mechanical Pipette – Bioendo Detail:
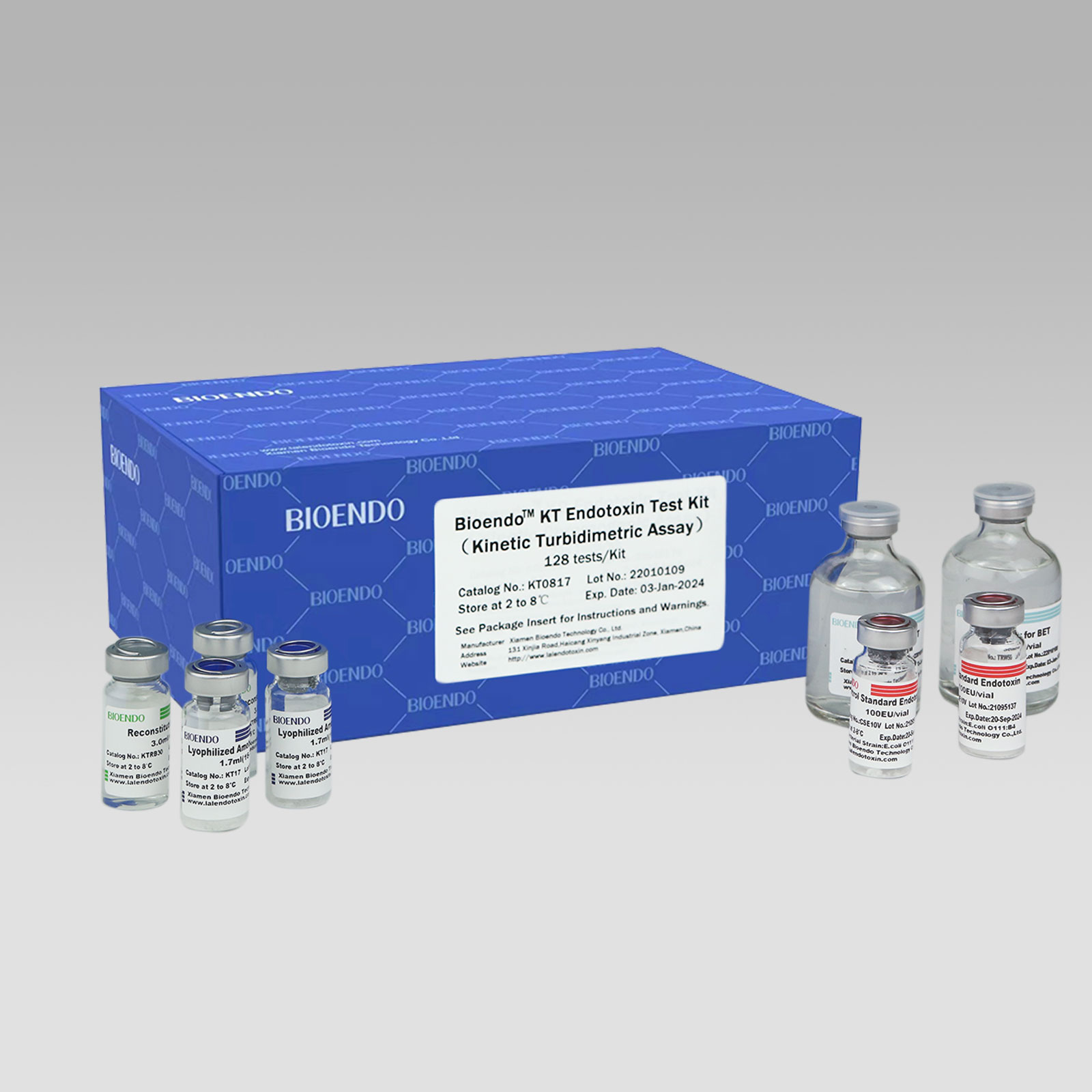
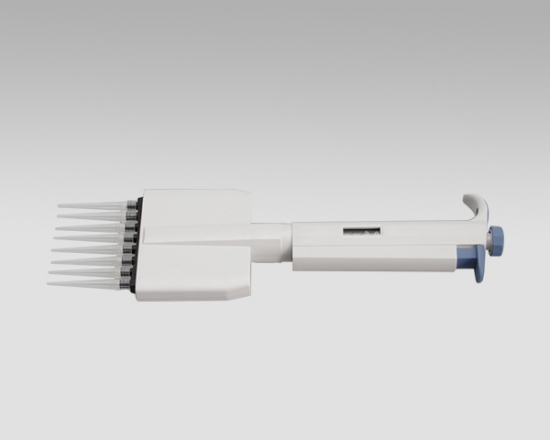
Eight-Channel Mechanical Pipettor
1. Product information
All multi-channel mechanical pipettor have been quality tested according to ISO8655-2:2002 with calibration certificate. The quality control involves gravimetric testing of each pipette with distilled water at 22℃. The multichannel mechanical pipettor is idea for the detectionof bacterial endotoxin lal endotoxin testing by kinetic turbidimetric andkinetic chromogenic method.
- Eight-Channel Mechanical Pipettor is available for standard 96-well plate
- Dispensing head rotates for optimum pipetting convenience
- Individual piston and tip cone assemblies allow easy repair and maintenance
- Compound material tip cone design allows visual seal verification
- Can be used with universal style pipette tips
-good for kinetic chromogenic, kinetic turbidimetric TAL orend-point chromogenic TAL endotoxin assay
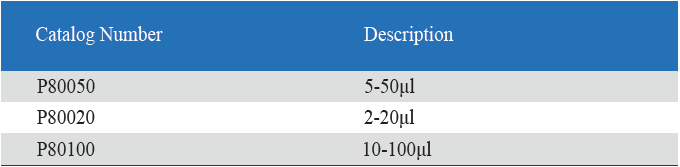
Product detail pictures:
Related Product Guide:
Our business puts emphasis over the administration, the introduction of talented staff, plus the construction of team building, attempting hard to boost the standard and liability consciousness of personnel customers. Our corporation successfully attained IS9001 Certification and European CE Certification of OEM Factory for BET Test Principle - Eight-channel Mechanical Pipette – Bioendo , The product will supply to all over the world, such as: Istanbul, South Korea, Las Vegas, To get more information about us as well as see all our products, please visit our website. To get more information please feel free to let us know. Thank you very much and wish your business always be great!
Hope that the company could stick to the enterprise spirit of "Quality, Efficiency, Innovation and Integrity", it will be better and better in the future.