OEM Manufacturer BET Test Procedure - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo
OEM Manufacturer BET Test Procedure - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo Detail:
Single-Channel Mechanical Pipettes (Semi-sterile)
1. Product Information
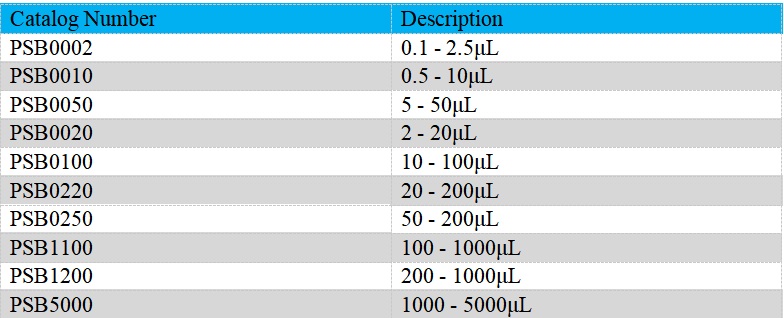
The Single-Channel Mechanical Pipettes (Semi-sterile) is adjustable, which could be used to dispense liquid precisely. Measuring volume of our Single-Channel Mechanical Pipettes (Semi-sterile) ranges from 0.1μL to 5mL. Products are produced based on ISO8655/DIN12650. It could be widely employed on endotoxin detection, etc..
2. Product Features
- Light weight, economic
- The pipettes cover volume range from 0.1μL to 5mL
- Semi-sterile
- Easy calibration and maintenance
Product detail pictures:

Related Product Guide:
"Quality first, Honesty as base, Sincere service and mutual profit" is our idea, in order to develop continuously and pursue the excellence for OEM Manufacturer BET Test Procedure - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo , The product will supply to all over the world, such as: French, Finland, Miami, Facing fierce global market competition, we have launched the brand building strategy and updated the spirit of "human-oriented and faithful service", with an aim to gain global recognition and sustainable development.
A nice supplier in this industry, after a detail and careful discussion, we reached a consensus agreement. Hope that we cooperate smoothly.







