OEM manufacturer BET Testing Method - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo
OEM manufacturer BET Testing Method - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo Detail:
Single-Channel Mechanical Pipettes (Semi-sterile)
1. Product Information
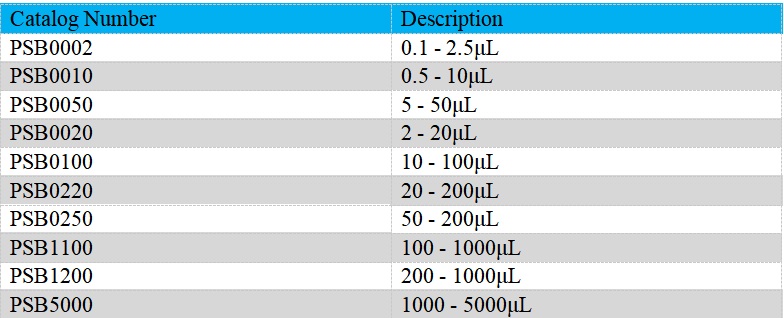
The Single-Channel Mechanical Pipettes (Semi-sterile) is adjustable, which could be used to dispense liquid precisely. Measuring volume of our Single-Channel Mechanical Pipettes (Semi-sterile) ranges from 0.1μL to 5mL. Products are produced based on ISO8655/DIN12650. It could be widely employed on endotoxin detection, etc..
2. Product Features
- Light weight, economic
- The pipettes cover volume range from 0.1μL to 5mL
- Semi-sterile
- Easy calibration and maintenance
Product detail pictures:

Related Product Guide:
With our rich experience and considerate services, we have been recognized as a reliable supplier for many international buyers for OEM manufacturer BET Testing Method - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo , The product will supply to all over the world, such as: America, El Salvador, Swaziland, We have been in operation for more than 10 years. We are dedicated to quality products and consumer support. We currently own 27 product utility and design patents. We invite you to visit our company for a personalized tour and advanced business guidance.
Production management mechanism is completed, quality is guaranteed, high credibility and service let the cooperation is easy, perfect!







