OEM manufacturer BET Testing Method - Single-Channel Mechanical Pipettor – Bioendo
OEM manufacturer BET Testing Method - Single-Channel Mechanical Pipettor – Bioendo Detail:
Single-Channel Mechanical Pipettor
1. Product Information
Single channel mechanical pipette is ideal tool to support endotoxin detection with Lyophilized Amebocyte Lysate which covers gel-clot technique, kinetic turbidimetric technique, kinetic chromogenic technique, and end-point chromogenic technique. All pipettors are produced by following ISO8655 – 2:2002. The quality control involves gravimetric testing of each pipette with distilled water at 22℃.
2. Product Features:
- Light weight, economic, low force design
- Measuring volume range from 0.1μL to 5mL
- Easy to calibrate and maintain with tool supplied
- Design helps to avoid repetitive strain injuries
- Calibrated in accordance with ISO8655. Each pipettor supplied with individual test certificate
- The low part is available for autoclaving
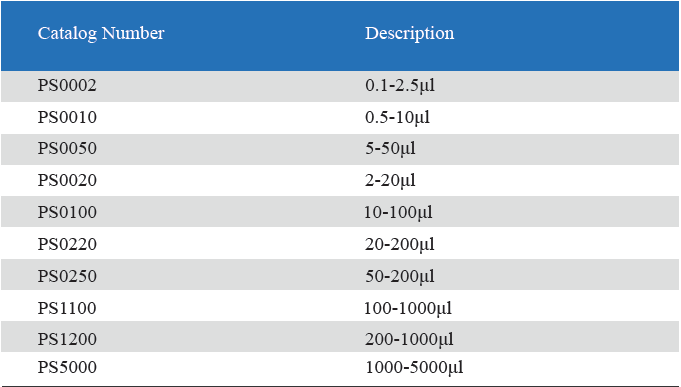
Product detail pictures:
Related Product Guide:
We not only will try our best to offer excellent services to every customer, but also are ready to receive any suggestion offered by our customers for OEM manufacturer BET Testing Method - Single-Channel Mechanical Pipettor – Bioendo , The product will supply to all over the world, such as: Ukraine, Bangkok, Belgium, we've got all day online sales to make sure the pre-sale and after-sale service in time. With all these supports, we can serve every customer with quality product and timely shipping with highly responsibility. Being a young growing company, we might not the best, but we are trying our best to be your good partner.
The factory workers have a good team spirit, so we received high quality products fast, in addition, the price is also appropriate, this is a very good and reliable Chinese manufacturers.