OEM Supply BET Test Method - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo
OEM Supply BET Test Method - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo Detail:
Single-Channel Mechanical Pipettes (Semi-sterile)
1. Product Information
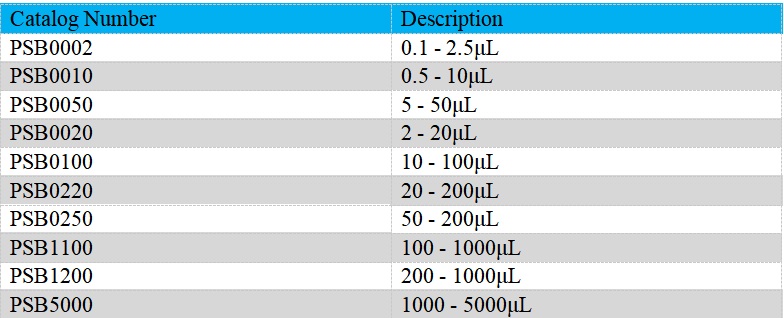
The Single-Channel Mechanical Pipettes (Semi-sterile) is adjustable, which could be used to dispense liquid precisely. Measuring volume of our Single-Channel Mechanical Pipettes (Semi-sterile) ranges from 0.1μL to 5mL. Products are produced based on ISO8655/DIN12650. It could be widely employed on endotoxin detection, etc..
2. Product Features
- Light weight, economic
- The pipettes cover volume range from 0.1μL to 5mL
- Semi-sterile
- Easy calibration and maintenance
Product detail pictures:

Related Product Guide:
It truly is a great way to improve our merchandise and repair. Our mission should be to create imaginative products to prospects with a excellent knowledge for OEM Supply BET Test Method - Single-Channel Mechanical Pipettes (Semi-sterile) – Bioendo , The product will supply to all over the world, such as: Morocco, Kuwait, Congo, We maintain long-term efforts and self-criticism, which helps us and improvement constantly. We strive to improve customer efficiency to save costs for customers. We do our best to improve the quality of product. We will not live up to the historic opportunity of the times.
The factory workers have rich industry knowledge and operational experience, we learned a lot in working with them,we are extremely grateful that we can encount a good company has excellent wokers.