OEM Supply BET Test Method - Single-Channel Mechanical Pipettor – Bioendo
OEM Supply BET Test Method - Single-Channel Mechanical Pipettor – Bioendo Detail:
Single-Channel Mechanical Pipettor
1. Product Information
Single channel mechanical pipette is ideal tool to support endotoxin detection with Lyophilized Amebocyte Lysate which covers gel-clot technique, kinetic turbidimetric technique, kinetic chromogenic technique, and end-point chromogenic technique. All pipettors are produced by following ISO8655 – 2:2002. The quality control involves gravimetric testing of each pipette with distilled water at 22℃.
2. Product Features:
- Light weight, economic, low force design
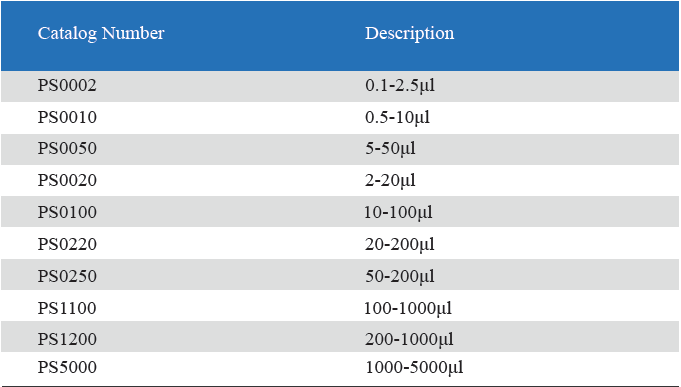
- Measuring volume range from 0.1μL to 5mL
- Easy to calibrate and maintain with tool supplied
- Design helps to avoid repetitive strain injuries
- Calibrated in accordance with ISO8655. Each pipettor supplied with individual test certificate
- The low part is available for autoclaving
Product detail pictures:

Related Product Guide:
Every single member from our higher effectiveness product sales staff values customers' requires and organization communication for OEM Supply BET Test Method - Single-Channel Mechanical Pipettor – Bioendo , The product will supply to all over the world, such as: St. Petersburg, Cairo, Bulgaria, Further, we are supported by highly experienced and knowledgeable professionals, who have immense expertise in their respective domain. These professionals work in close coordination with each other to offer our clients an effective range of products.
The company comply with the contract strict, a very reputable manufacturers, worthy a long-term cooperation.







