OEM/ODM China Bacterial Endotoxin Test Gel Clot Method Sop - Bioendo GC Endotoxin Test Kit (Gel Clot Assay) – Bioendo
OEM/ODM China Bacterial Endotoxin Test Gel Clot Method Sop - Bioendo GC Endotoxin Test Kit (Gel Clot Assay) – Bioendo Detail:
Bioendo GC Endotoxin Test Kit
Bioendo GC Endotoxin Test Kit (Gel Clot Assay)
1. Product Information
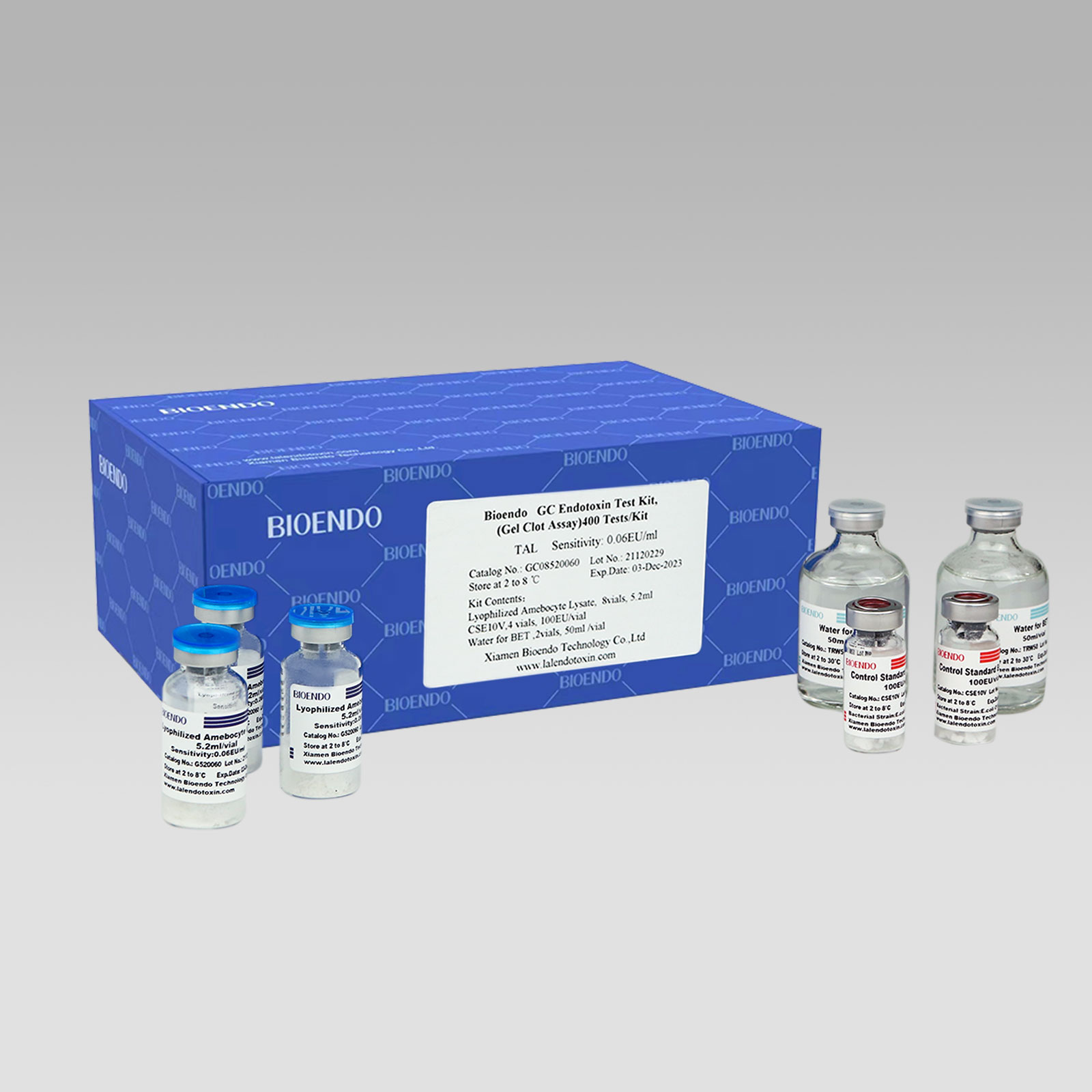
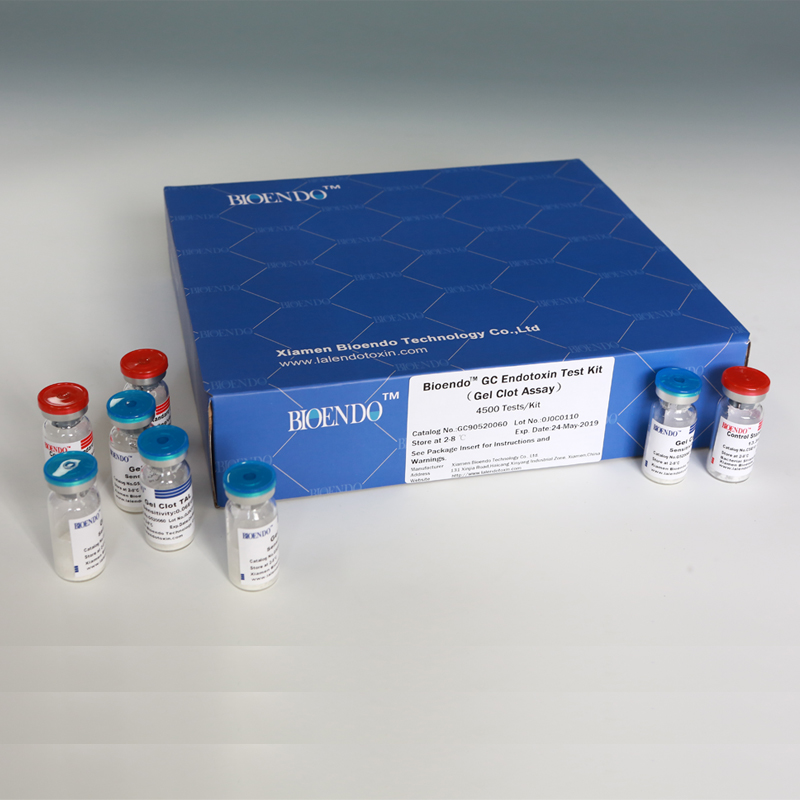
Bioendo GC Endotoxin Test Kit (Gel Clot Assay) contains 128 Tests/Kit, 400Tests/Kit, 1600Tests/Kit, and 4500 Tests/Kit. And sensitivity of Amebocyte Lysate included by the Kit is 0.03 EU/ml, 0.06 EU/ml, 0.125 EU/ml, 0.25 EU/ml, 0.5 EU/ml. The Kit contains Amebocyte Lysate, CSE and Water for BET. Four kinds of configuration of gel-clot endotoxin assay kit to satisfied the demand. This means you could choose different configuration kits according to the requirement.
2. Product Parameter
Sensitivities: 0.03EU/ml, 0.06EU/ml, 0.125EU/ml, 0.25EU/ml, 0.5 EU/ml
3.Product Feature and Application
Full range of sensitivities to meet the demand. No special instrument is needed for endotoxin detection with Bioendo GC Endotoxin Test Kit (Gel Clot Assay). It is the best choice when you need to do lots of endotoxin detection.
Note:
Lyophilized Amebocyte Lysate (LAL/TAL) reagent manufactured by Bioendo is made from amebocyte lysate derived blood of horseshoe crab.
|
Catalog No. |
Sensitivity (EU/ml) |
Description |
Kit Contents |
|
GC90520030 |
0.03 |
Bioendo™ GC Endotoxin Test Kit (Gel Clot Assay) 4500 Tests/Kit |
90 Gel Clot Lyophilized Amebocyte Lysate, 5.2ml (50 Tests/Vial); 20 CSE10V; |
|
GC90520060 |
0.06 |
||
|
GC90520125 |
0.125 |
||
|
GC90520250 |
0.25 |
||
|
GC90520500 |
0.5 |
||
|
GC08520030 |
0.03 |
Bioendo™ GC Endotoxin Test Kit (Gel Clot Assay) 400 Tests/Kit |
8 Gel Clot Lyophilized Amebocyte Lysate, 5.2ml (50 Tests/Vial); 4 CSE10V; 2 Water for BET, 50ml/vial; |
|
GC08520060 |
0.06 |
||
|
GC08520125 |
0.125 |
||
|
GC08520250 |
0.25 |
||
|
GC08520500 |
0.5 |
||
|
GC100170030 |
0.03 |
Bioendo™ GC Endotoxin Test Kit (Gel Clot Assay) 1600 Tests/Kit |
100 Gel Clot Lyophilized Amebocyte Lysate, 1.7ml (16 Tests/Vial); 10 CSE10V; |
|
GC100170060 |
0.06 |
||
|
GC100170125 |
0.125 |
||
|
GC100170250 |
0.25 |
||
|
GC100170500 |
0.5 |
||
|
GC08170030 |
0.03 |
Bioendo™ GC Endotoxin Test Kit (Gel Clot Assay) 128 Tests/Kit |
8 Gel Clot Lyophilized Amebocyte Lysate, 1.7ml (16 Tests/Vial); 4 CSE10V; 2 Water for BET, 50ml/vial; |
|
GC08170060 |
0.06 |
||
|
GC08170125 |
0.125 |
||
|
GC08170250 |
0.25 |
||
|
GC08170500 |
0.5 |
Product condition:
Endotoxin Test kit’s sensitivity and the Control Standard Endotoxin potency are assayed against USP Reference Standard Endotoxin. The Endotoxin Test reagentkits come with product instruction, Certificate of Analysis.
Product detail pictures:

Related Product Guide:
We are going to dedicate ourselves to providing our esteemed buyers together with the most enthusiastically thoughtful products and services for OEM/ODM China Bacterial Endotoxin Test Gel Clot Method Sop - Bioendo GC Endotoxin Test Kit (Gel Clot Assay) – Bioendo , The product will supply to all over the world, such as: Philippines, Jamaica, Bolivia, We attained ISO9001 which provides solid foundation for our further development. Persisting in "High quality, Prompt Delivery, Competitive Price", we have established long-term cooperation with clients from both overseas and domestically and get new and old clients' high comments. It is our great honor to meet your demands. We are sincerely expecting your attention.
This company has the idea of "better quality, lower processing costs, prices are more reasonable", so they have competitive product quality and price, that's the main reason we chose to cooperate.







