OEM/ODM China Bacterial Endotoxin Test Gel Clot Method Sop - Gel Clot Lyophilized Amebocyte Lysate Single Test in Ampoule – Bioendo
OEM/ODM China Bacterial Endotoxin Test Gel Clot Method Sop - Gel Clot Lyophilized Amebocyte Lysate Single Test in Ampoule – Bioendo Detail:
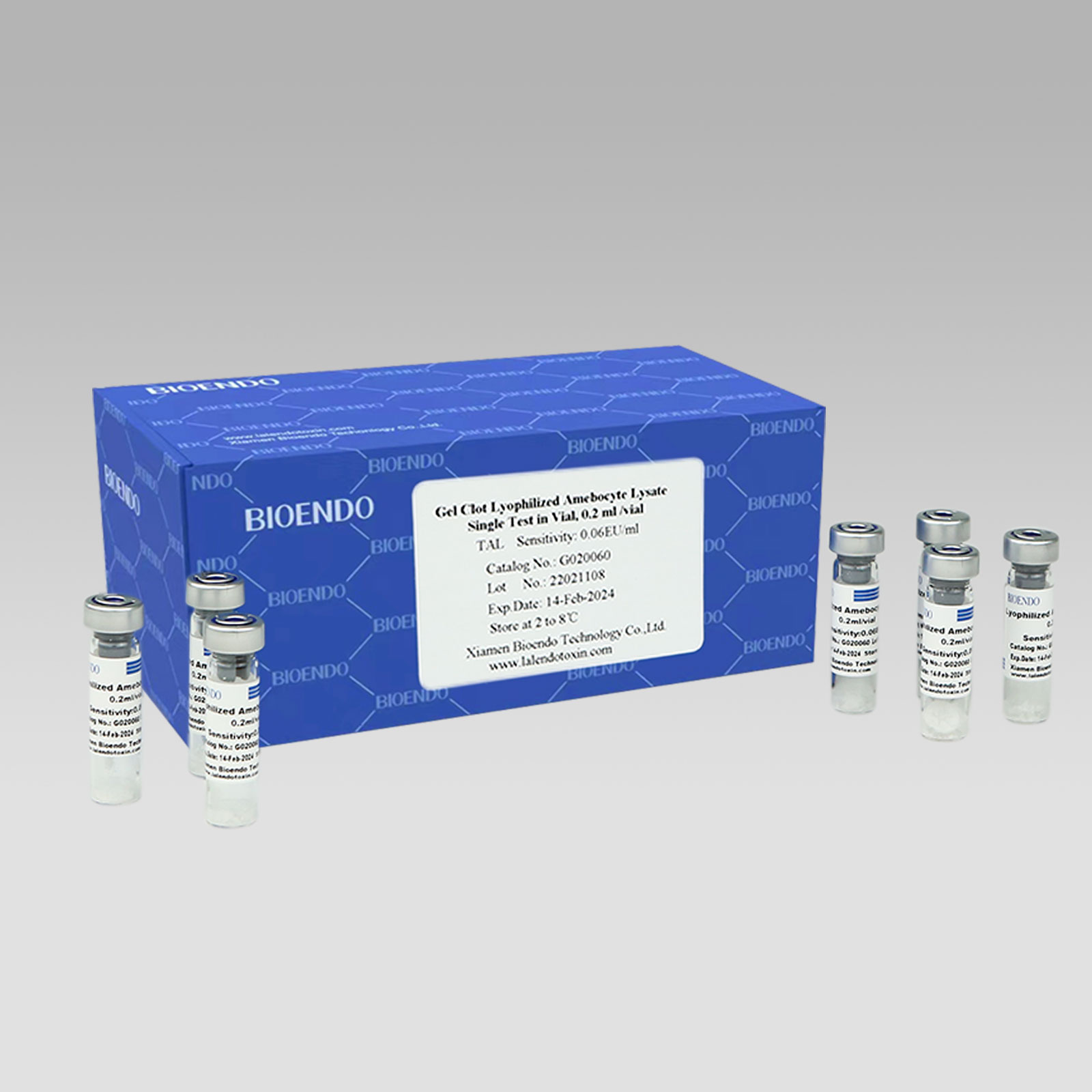
Gel Clot Lyophilized Amebocyte Lysate Single Test in Ampoule
1. Product Information
Gel Clot Lyophilized Amebocyte Lysate Single Test in ampoule Contain endotoxin-specific Amebocyte Lysate which includes beta-glucan inhibitor in the formulation and will not react to beta-glucan. For our Single Test in glass ampoule, you could add sample to the glass ampoules directly. This means you don’t need to reconstitute Amebocyte Lysate at first, and you will decide how many tests use each time to avoid waste. Endotoxin free tubes for the dilution of Lyophilized CSE is necessary. Operation of endotoxin detection use Bioendo Gel Clot Lyophilized Amebocyte Lysate Single Test in ampoule conforms to the national Pharmacopoeia.
2. Product Parameter
Gel clot assay single test glass ampoule.
Sensitivities: 0.03EU/ml, 0.06EU/ml, 0.125EU/ml, 0.25 EU/ml, 0.5EU/ml.
3.Product Feature and Application
Single step endotoxin detection,
Normal water bath or dry heat incubator is available for the gel clot method.
Suitable for end-product endotoxin testing before product released.
Product sensitivity standardized according to the China Pharmacopoeia criterion.
Note:
Lyophilized Amebocyte Lysate (LAL) reagent manufactured by Bioendo is made from lysate of amebocytes (white blood cells) from the horseshoe crab.
|
Catalog No. |
Sensitivity EU/ml |
Description |
Kit Contents |
|
GS010030 |
0.03 |
Bioendo Gel Clot Endotoxin Test Kit, Single Test in Ampoule. |
10 tests per pack |
|
GS010060 |
0.06 |
||
|
GS010125 |
0.125 |
||
|
GS010250 |
0.25 |
||
|
GS010500 |
0.5 |
Product Condition
The sensitivity of Lyophilized Amebocyte Lysate and potency of Control Standard Endotoxin are assayed against USP Reference Standard Endotoxin. The Lyophilized Amebocyte Lysate reagent kits come with product instruction, Certificate of Analysis.
Why the most selected gel clot assay kit G01:
1. The most economical and commonly used test reagent for endotoxin detection.
2. Single test in a ampoule for one step reduce the risk of contamination.
3. Gel clot assay single test glass ampoule no need sophisticated microplate reader.
4. Saving endotoxin free tube when using G01 series to test endotoxins in the procedure of endotoxin test assay’s operation.
Product detail pictures:


Related Product Guide:
Our organization has been focusing on brand strategy. Customers' gratification is our greatest advertising. We also source OEM provider for OEM/ODM China Bacterial Endotoxin Test Gel Clot Method Sop - Gel Clot Lyophilized Amebocyte Lysate Single Test in Ampoule – Bioendo , The product will supply to all over the world, such as: Libya, Belgium, Puerto Rico, We honor ourselves as a company that comprises of a strong team of professionals who are innovative and well experienced in the international trading, business development and product advancement. Moreover, the company stays unique among its competitors due to its superior standard of quality in production, and its efficiency and flexibility in business support.
It is a very good, very rare business partners, looking forward to the next more perfect cooperation!