OEM/ODM China Dry heat sterilizer validation by ECV - Eight-channel Mechanical Pipette – Bioendo
OEM/ODM China Dry heat sterilizer validation by ECV - Eight-channel Mechanical Pipette – Bioendo Detail:
Eight-Channel Mechanical Pipettor
1. Product information
All multi-channel mechanical pipettor have been quality tested according to ISO8655-2:2002 with calibration certificate. The quality control involves gravimetric testing of each pipette with distilled water at 22℃. The multichannel mechanical pipettor is idea for the detectionof bacterial endotoxin lal endotoxin testing by kinetic turbidimetric andkinetic chromogenic method.
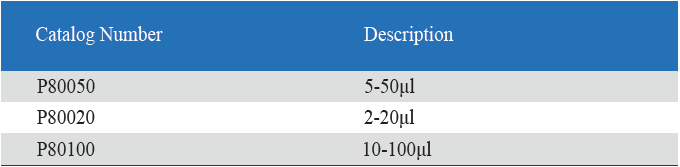
- Eight-Channel Mechanical Pipettor is available for standard 96-well plate
- Dispensing head rotates for optimum pipetting convenience
- Individual piston and tip cone assemblies allow easy repair and maintenance
- Compound material tip cone design allows visual seal verification
- Can be used with universal style pipette tips
-good for kinetic chromogenic, kinetic turbidimetric TAL orend-point chromogenic TAL endotoxin assay
Product detail pictures:

Related Product Guide:
Our company since its inception, constantly regards product or service high quality as business life, continually improve creation technology, make improvements to product high-quality and consistently strengthen business total high-quality management, in strict accordance together with the national standard ISO 9001:2000 for OEM/ODM China Dry heat sterilizer validation by ECV - Eight-channel Mechanical Pipette – Bioendo , The product will supply to all over the world, such as: Nigeria, St. Petersburg, Chile, We believe in quality and customer satisfaction achieved by a team of highly dedicated individuals. The team of our company with the use of cutting-edge technologies delivers impeccable quality products supremely adored and appreciated by our customers worldwide.
As an international trading company, we have numerous partners, but about your company, I just want to say, you are really good, wide range, good quality, reasonable prices, warm and thoughtful service, advanced technology and equipment and workers have professional training, feedback and product update is timely, in short, this is a very pleasant cooperation, and we look forward to the next cooperation!






