OEM/ODM China Dry heat sterilizer validation by ECV - Rapid Gel Clot 10 Samples Kit – Bioendo
OEM/ODM China Dry heat sterilizer validation by ECV - Rapid Gel Clot 10 Samples Kit – Bioendo Detail:
Bioendo Rapid Gel Clot Endotoxin Assay Kit is designed to rapidly quantify endotoxin in water or dialysate. Generally, RG kit’s result could be gained within 30 minutes. Under the guidance of detecting endotoxin in water or dialysate quickly, endotoxin detection with Bioendo Rapid Gel Clot Endotoxin Assay Kit does not need the multi steps’ dilution of Control Standard Endotoxin and test samples. Operation procedures are very convenient, additional experimental equipment are required. It is a convenient and rapid way to detect endotoxin in especial suitable for water or dialysate.
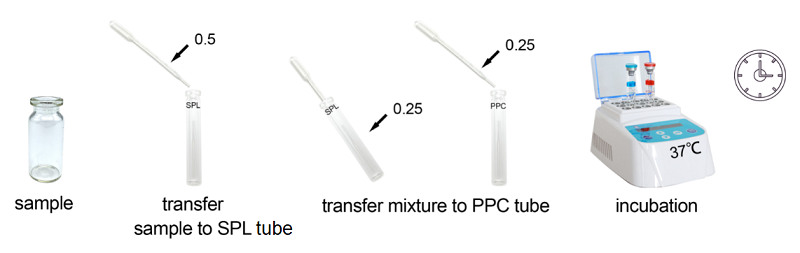
2. Product Parameter
Sensitivity Range: 0.03EU/ml, 0.06EU/ml, 0.125EU/ml, 0.25EU/ml, 0.5EU/ml
10 sample tests in the kit.
Assay time: less than 30 minutes
3. Product Application
Bioendo Rapid Gel Clot Endotoxin Assay Kit is designed to rapidly quantify endotoxin in water or dialysate as well as do quickly endotoxin detection in life science research.
Note:
Lyophilized Amebocyte Lysate (LAL) reagent manufactured by Bioendo is made from amebocyte lysate derived blood of horseshoe crab.
|
Catalog No. |
Description |
Kit Contents |
Sensitivity EU/ml |
Reaction Time minutes |
|
RG10025003 |
BioendoTM Rapid Gel Clot Endotoxin Assay Kit, 10 Samples Kit |
10 SPL Tubes; 10 PPC Tubes; 10 Endotoxin-free Sample Bottles; 10 Packs of (3pcs Transfer Pipettes) |
0.03 |
≤60 |
|
RG10025006 |
0.06 |
≤60 |
||
|
RG100250125 |
0.125 |
≤45 |
||
|
RG10025025 |
0.25 |
≤30 |
||
|
RG10025050 |
0.5 |
≤30 |
Product detail pictures:



Related Product Guide:
Dedicated to strict quality control and thoughtful customer service, our experienced staff members are always available to discuss your requirements and ensure full customer satisfaction for OEM/ODM China Dry heat sterilizer validation by ECV - Rapid Gel Clot 10 Samples Kit – Bioendo , The product will supply to all over the world, such as: panama, Suriname, Provence, Since the establishment of our company, we've realized the importance of providing good quality goods and the best before-sales and after-sales services. Most problems between global suppliers and clients are due to poor communication. Culturally, suppliers can be reluctant to question points they do not understand. We break down these barriers to ensure you get what you want to the level you expect, when you want it.
The factory technical staff not only have high level of technology, their English level is also very good, this is a great help to technology communication.







