OEM/ODM Factory Application Of LAL Test - Eight-channel Mechanical Pipette – Bioendo
OEM/ODM Factory Application Of LAL Test - Eight-channel Mechanical Pipette – Bioendo Detail:
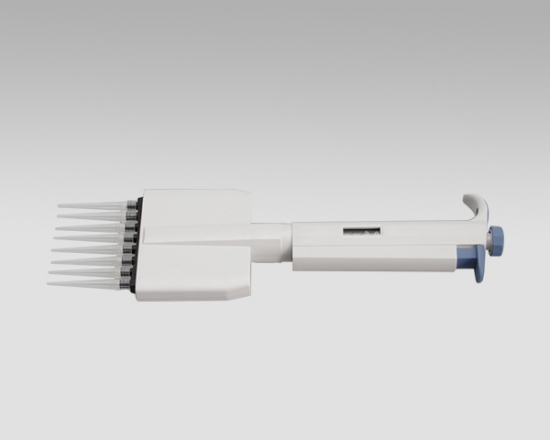
Eight-Channel Mechanical Pipettor
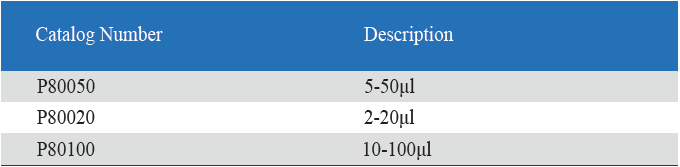
1. Product information
All multi-channel mechanical pipettor have been quality tested according to ISO8655-2:2002 with calibration certificate. The quality control involves gravimetric testing of each pipette with distilled water at 22℃. The multichannel mechanical pipettor is idea for the detectionof bacterial endotoxin lal endotoxin testing by kinetic turbidimetric andkinetic chromogenic method.
- Eight-Channel Mechanical Pipettor is available for standard 96-well plate
- Dispensing head rotates for optimum pipetting convenience
- Individual piston and tip cone assemblies allow easy repair and maintenance
- Compound material tip cone design allows visual seal verification
- Can be used with universal style pipette tips
-good for kinetic chromogenic, kinetic turbidimetric TAL orend-point chromogenic TAL endotoxin assay
Product detail pictures:
Related Product Guide:
We are also focusing on enhancing the things administration and QC program in order that we could keep fantastic advantage within the fiercely-competitive enterprise for OEM/ODM Factory Application Of LAL Test - Eight-channel Mechanical Pipette – Bioendo , The product will supply to all over the world, such as: United Kingdom, Marseille, Sweden, Company name, is always regarding quality as company' s foundation, seeking for development via high degree of credibility , abiding by ISO quality management standard strictly, creating top-ranking company by spirit of progress-marking honesty and optimism.
High production efficiency and good product quality, fast delivery and completed after-sale protection, a right choice, a best choice.







