OEM/ODM Factory Application Of LAL Test - Single-Channel Mechanical Pipettor – Bioendo
OEM/ODM Factory Application Of LAL Test - Single-Channel Mechanical Pipettor – Bioendo Detail:
Single-Channel Mechanical Pipettor
1. Product Information
Single channel mechanical pipette is ideal tool to support endotoxin detection with Lyophilized Amebocyte Lysate which covers gel-clot technique, kinetic turbidimetric technique, kinetic chromogenic technique, and end-point chromogenic technique. All pipettors are produced by following ISO8655 – 2:2002. The quality control involves gravimetric testing of each pipette with distilled water at 22℃.
2. Product Features:
- Light weight, economic, low force design
- Measuring volume range from 0.1μL to 5mL
- Easy to calibrate and maintain with tool supplied
- Design helps to avoid repetitive strain injuries
- Calibrated in accordance with ISO8655. Each pipettor supplied with individual test certificate
- The low part is available for autoclaving
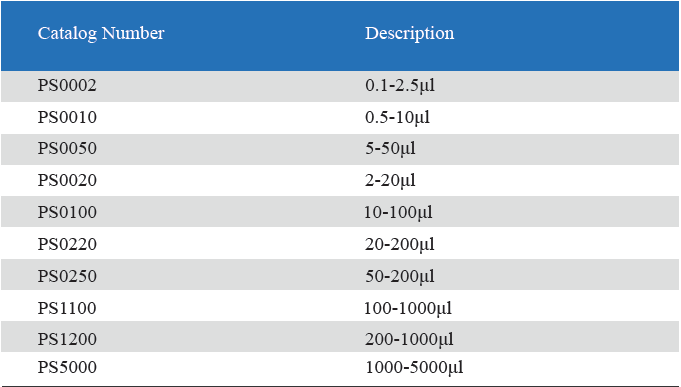
Product detail pictures:

Related Product Guide:
The corporate upholds the philosophy of "Be No.1 in excellent, be rooted on credit rating and trustworthiness for growth", will keep on to serve outdated and new clients from home and abroad whole-heatedly for OEM/ODM Factory Application Of LAL Test - Single-Channel Mechanical Pipettor – Bioendo , The product will supply to all over the world, such as: Liberia, Sacramento, Chicago, We always insist on the principle of "Quality and service are the life of the product". Till now, our products have been exported to more than 20 countries under our strict quality control and high level service.
The company account manager has a wealth of industry knowledge and experience, he could provide appropriate program according our needs and speak English fluently.